Fed‑Batch PHB Production from Glycerol using the LAMBDA Minifor Fermenter-Bioreactor
Polyhydroxybutyrate (PHB) is a biodegradable bioplastic produced intracellularly by many bacterial species when an excess of carbon is present under nitrogen-limited conditions. Due to its biodegradability and material properties comparable to conventional plastics, PHB is widely studied as a sustainable alternative to petroleum-based polymers.
In a recent study conducted at Universidad Autónoma Metropolitana (Mexico), researchers investigated PHB production from glycerol, a major by-product of biodiesel production, using controlled fed-batch fermentation. To ensure reproducible cultivation and stable process conditions, the experiments were performed using the LAMBDA Minifor laboratory fermenter. This allowed PHB synthesis to be studied under well-defined environmental and nutritional conditions.
Reference: León Santiesteban, H. H., Aguirre Aguilar, J., Beltrán, D. Á., Contreras Larios, J. L., Reyes Chilpa, R., García Martínez, J. C., & González Brambila, M. M. (2026). Bioplastic Production in Circular Economy Paths with Glycerol and Whey. Catalysts, 16(2), 178. doi.org/10.3390/catal16020178
Use of the LAMBDA Minifor Fermenter
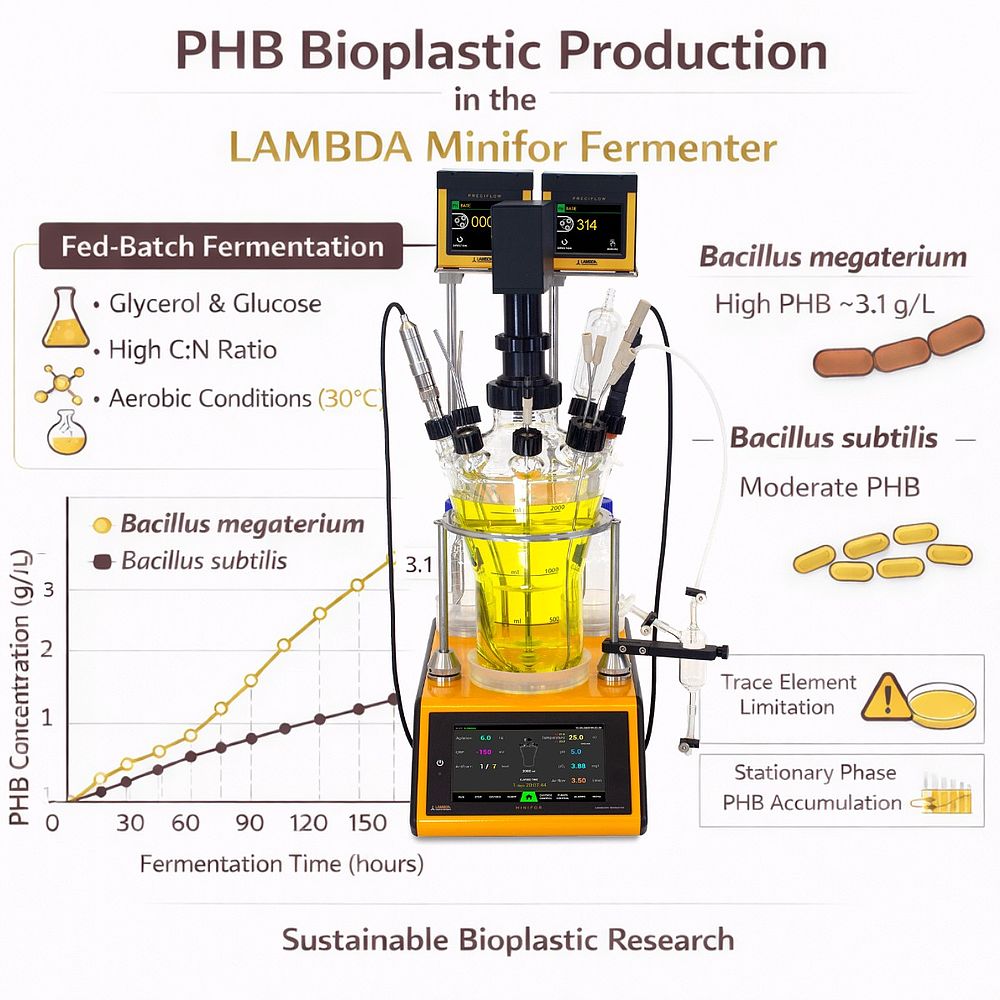
The LAMBDA Minifor fermenter was used as the primary bioreactor for the cultivation of Bacillus megaterium and Bacillus subtilis, two known PHB-producing microorganisms. Each strain was cultivated separately under identical fed-batch fermentation conditions, enabling direct comparison of PHB production performance.
The Minifor enabled stable control of essential fermentation parameters, including:
Temperature control at 30 °C
Continuous aerobic cultivation
Gentle mixing to maintain homogeneous conditions
Long-duration fed batch fermentation runs up to 180 hours
Operation under a high carbon-to-nitrogen (C:N) ratio, promoting intracellular PHB accumulation
These controlled conditions are critical for studying bioplastic production, as PHB synthesis occurs primarily when microbial growth slows and excess carbon is redirected toward polymer storage.
Fermentation Method
Fed-batch fermentation was performed using the LAMBDA Minifor laboratory fermenter with a working volume of approximately 1.7 L.
The culture medium contained:
Glycerol and glucose as carbon sources
Ammonium sulfate as a limiting nitrogen source
A high C:N ratio (~210–220:1) to induce PHB synthesis
Cultivation was carried out under controlled aerobic conditions at 30 °C. Separate fermentations were performed using Bacillus megaterium and Bacillus subtilis.
Additional experiments were conducted with and without trace elements in the medium. Limiting trace elements increased metabolic stress, which promoted intracellular PHB production rather than continued biomass growth.
Samples were collected during fermentation to monitor biomass concentration, substrate consumption, and PHB production.
Results
Both bacterial strains successfully produced PHB bioplastic under the controlled conditions provided by the LAMBDA Minifor fermenter.
Bacillus megaterium showed the highest productivity, reaching PHB concentrations of approximately 3.1 g L⁻¹. PHB accumulation occurred mainly during the stationary phase, when microbial growth slowed and carbon was redirected toward polymer synthesis.
Bacillus subtilis also produced PHB under the same fed-batch fermentation conditions, although at lower concentrations. As with B. megaterium, PHB synthesis occurred primarily after active growth had slowed.
Limiting trace elements increased PHB production in both strains by promoting intracellular polymer accumulation rather than biomass formation.
The controlled fermentation environment provided by the Minifor ensured reproducible results and enabled reliable comparison between microbial strains.
Conclusion
The LAMBDA Minifor laboratory fermenter provides a reliable platform for fed-batch PHB production, bioplastic research, and microbial fermentation studies. Its ability to maintain stable temperature, aeration, and mixing conditions over extended cultivation periods allows reproducible intracellular biopolymer production.
The system is well suited for applications including:
PHB and bioplastics research
Fed-batch fermentation process development
Microbial strain evaluation
- Laboratory-scale bioprocess optimization
Take your research to the next level with the LAMBDA Minifor2Bio touch Fermenter-Bioreactor. Discover its advanced capabilities and how it can enhance your workflow. Learn more on our product page:
www.lambda-instruments.com/fermenter-bioreactor-touch/
NEW! The latest Minifor2Bio touch laboratory fermenters-bioreactors are now available.
Read more: fermenters.eu
For inquiries, quotations, or pricing information, please contact us at sales@lambda-instruments.com.