Monoclonal Antibody Production from Continuous Hybridoma Culture for 100 Days in the LAMBDA MINIFOR Bioreactor
Continuous bioprocessing is rapidly becoming the preferred strategy for achieving higher productivity, consistent product quality, and reduced operational costs in modern biomanufacturing. To enable this shift, researchers require a laboratory bioreactor capable of cultivating sensitive mammalian cells under stable conditions for long periods. A recent independent study conducted at the Center for Genetic Engineering and Biotechnology (CIGB), Cuba, demonstrated that the LAMBDA MINIFOR bioreactor offers exceptional long-term stability and precision for mammalian cell cultivation, successfully supporting a continuous 100-day hybridoma culture in protein-free medium.
Reference: González M., Aragón H., Medel M., Hernández D., Ferro W., et al. (2024).
Continuous culture of mouse hybridoma cells for 100 Days in the LAMBDA MINIFOR bioreactor.
Biotecnología Aplicada, 41(2): 2211–2218.
Study Overview
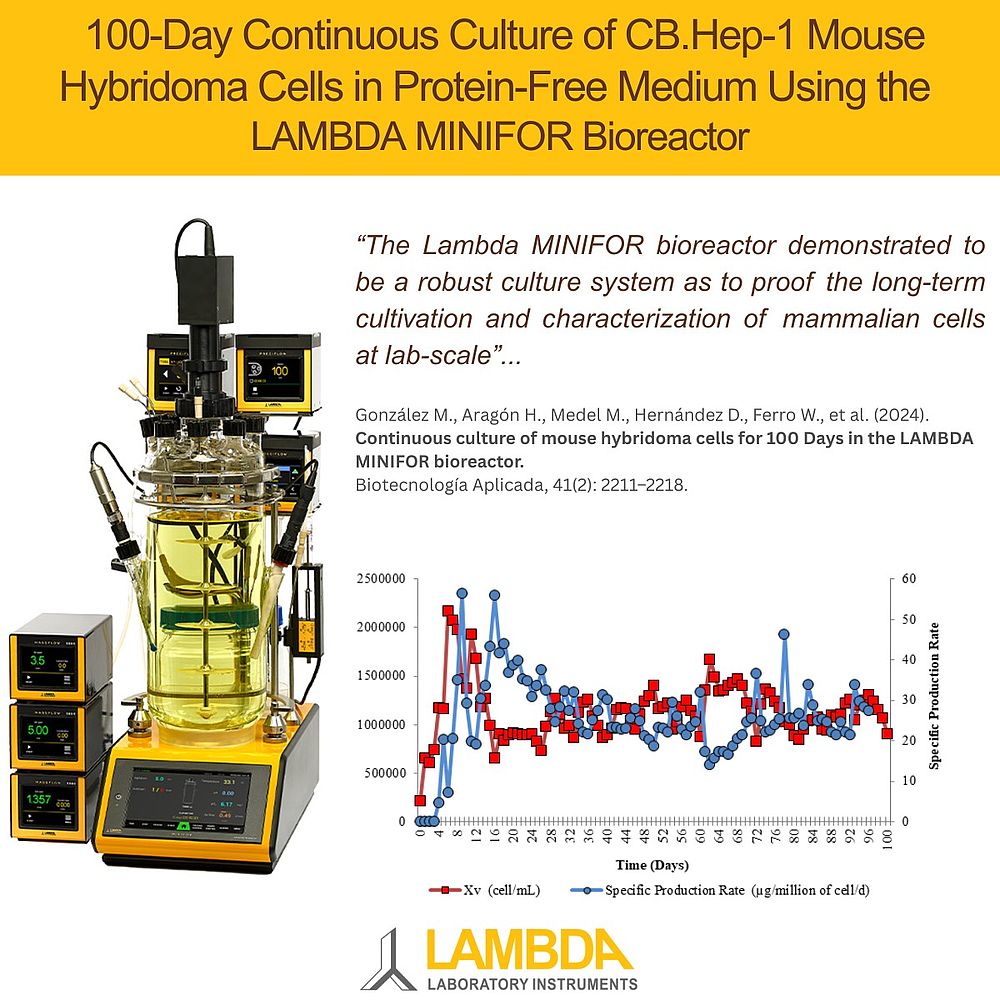
In this study, CB.Hep-1 hybridoma cells were cultured continuously in the MINIFOR bioreactor under controlled conditions designed to maintain gentle, stable growth. The bioreactor operated at 36–37 °C with a pH of 6.8–7.2, using LAMBDA’s unique biomimetic fishtail agitation to minimize shear stress. Aeration was maintained at low levels without requiring CO₂ supplementation, and the system handled daily medium renewal with no performance drift or contamination events. This setup enabled the hybridoma cells to be cultured reliably over a full 100 days.
Hybridoma-based mAb production, although historically robust, can be prone to genetic drift and productivity loss under prolonged culture conditions. Therefore, maintaining cell line stability and product quality over extended periods is a critical benchmark for evaluating bioreactor performance.
Continuous operation began on Day 6 and was maintained for 100 days with up to 19% daily volume replacement, ensuring steady-state conditions.
Performance Results
Throughout the entire operation, the LAMBDA MINIFOR maintained high and consistent cell performance. Average viability was 82.5% with a stable viable cell density of 1.1 × 10⁶ cells/mL.
Monoclonal antibody production remained steady with a mean concentration of 122.7 mg/L and a specific productivity of 25.7 µg per million cells per day.
Overall, the culture produced 12.5 g of monoclonal antibody, from which 10.1 g of highly purified protein was recovered. Product quality remained exceptional, with purity over 99%, no aggregation, and unchanged antigen-binding specificity across the entire production period.
Product Quality and Stability
One of the most significant findings of the study was that extended continuous operation did not negatively affect the molecular quality or biochemical characteristics of the monoclonal antibody.
The purified product retained full structural integrity, maintained its affinity constant, and showed no degradation or variation in immunological behavior. This highlights the suitability of the MINIFOR for applications where long-term product consistency is essential, such as hybridoma-based monoclonal antibody production and continuous perfusion processes.
Scale-Up Potential
Based on MINIFOR data, the researchers modeled large-scale continuous operation:
| Scale | Operation Mode | Estimated Annual Yield |
|---|---|---|
| 600 L (no cell retention) | Continuous | 2,025 g mAb/year |
| 50 L (with cell retention) | Continuous | 1,378 g mAb/year |
Such scalability underscores the MINIFOR’s role as an accurate and economical scale-down model for mammalian cell culture optimization—enabling cost-effective transition to pilot and production bioreactors.
Conclusion
The 100-day CB.Hep-1 hybridoma study provides strong evidence that the LAMBDA MINIFOR bioreactor is an exceptionally capable platform for long-term mammalian cell culture and continuous bioprocess development.
Its stable operational performance, precise environmental control, gentle mixing, and consistent product quality make it an ideal solution for labs working on monoclonal antibody production, continuous culture optimization, and advanced bioprocess research. The results positions the MINIFOR as one of the most reliable and efficient bench-scale bioreactors for continuous mammalian cell cultivation.

Interested in integrating the LAMBDA MINIFOR or Minifor2Bio touch Bioreactor in your lab? Contact us for technical details, configuration options, or to arrange a Teams/Zoom meetings.
Email us: sales@lambda-instruments.com