Frequently scientists want to know how their culture grows and what its metabolic activity during biotransformation is. For that reason, they are looking for instruments which can measure the optical density (OD) of the culture.
Optical density is a logarithmic function and increasing the number of light absorption unit by one means that the intensity of light passing through the sample has diminished 10 times! Needless to say that at an optical density of merely 4 the light intensity has decreased by a factor of 10 000. It is a demanding task for electronics to measure such a low signal with precision. And what about OD 20 or 100? Even though many devices for the measurement of optical density of culture broths have been presented, none are really satisfactory.
There is much interference during such a measurement. The first and most problematic is that OD measurement also measures dead cells. If many dead cells are present in the culture the resulting metabolic activity will be wrong. Also small air bubbles are measured and counted as living cells! The number of microscopic air bubbles, especially in dense cultures, may be quite high. No need to say that any precipitation or coloration formed during the culture will distort the estimation of metabolic activity of the measured culture.
Determination of cell growth
Cell growth measurement is usually done by taking samples at different times:
1. Staining and counting of dead and alive cells (microscope) in case of mammalian cells
2. Total cell density (dead and alive) by OD 600-650nm in photometer for bacteria/yeasts or total cells density (dead and alive) by counting in a Neubauer cell counting chamber under the microscope
3. Alive cells by dilution of sample, incubation of 0.1ml on agar plate or incubation of 1 ml in agar plate for 1-3 days at growth temperature in incubator, used in the case of bacteria/yeast
4. By a “coulter counter” cell or particle counting system
In situ measurements
LAMBDA opens a new possibility to access the cell growth kinetics by measuring and recording amounts of acid or base necessary to keep the pH of the culture constant.
The growth of the culture is globally seen as a large complex of biochemical or chemical reactions, which use substrates and yield products in the form of the desired product or/and cell mass.
Cell growth always produces CO2 which is acidic and to keep the pH constant, this acid (which partially escapes) has to be compensated by the addition of another acid by a pH controller with an acid pump. (The formation of metabolic acids and bases other than CO2 are of course also taken into account by this measurement globally).
In balanced regular growth the production of CO2 and the need of pH correcting acid is proportional to the growth (as the sum of all metabolic processes in the cell). Therefore, the amount of added pH correction solution (acid or base) is a precise indication of the growth extent of the culture. The controlled addition of acid equals in principle to an analytical titration and is therefore very precise.
The LAMBDA method using the INTEGRATOR is strictly connected with the real metabolic activity
The LAMBDA PUMP-FLOW INTEGRATOR transforms the impulses driving the motor of the pump into a voltage which can be recorded either on a recorder or shown on a screen by a PC using the fermentation software (FNet or SIAM).
The quantification of the INTEGRATOR signal can be easily obtained by using acids of known concentration and by calibration of the pump flow. This is easily done. Then, this value is correlated to the growth extent measured by another suitable growth rate method. Afterwards, the mere signal of the PUMP-FLOW INTEGRATOR allows precise and very inexpensive real growth kinetics. This is not so for many very expensive methods of growth rate measurement, e.g. the measurement of optical density (OD) of the culture. Such a measurement is not precise because also dead cells, air bubbles, precipitates and any other color or turbidity formation appear as an increase in cell concentration.
The trace obtained by the INTEGRATOR gives also indication about growth problems, contamination and the extent of the biotransformation. It is an important parameter when series of cultures have to be compared. It would be pity for any scientist not to use this easy and precise system for the following of the cell growth either in eukaryotic or prokaryotic cultures.
The pH trace obtained by the majority of competing bioreactors is almost always just a straight line saying nothing about the culture. However, if the required quantity of acid or base to keep the pH constant during the whole culture growth is visualized by the INTEGRATOR, this opens a new dimension full of valuable information for the scientist.
Below is one example of the trace of an acid pump activity transformed by the INTEGRATOR during one biotransformation culture (red trace with two resets). The red trace is the only one which is clearly exponential, as it should be. By totalizing the consumption of acid, the extent of transformation and the immediate state of the culture can be derived. No need to say how important such information can be for the control and reproducibility of different cultures.
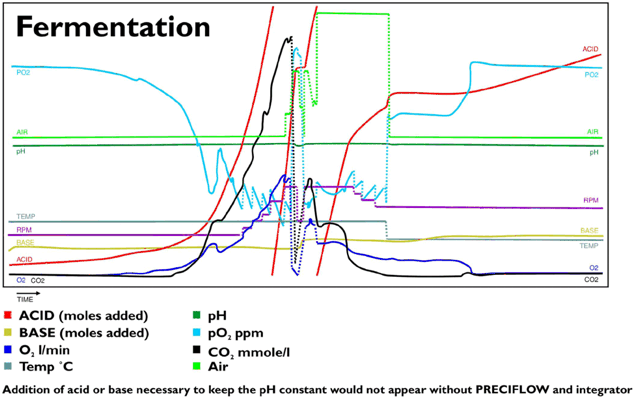
Clients of LAMBDA are so convinced about the usefulness of the INTEGRATOR, that they put INTEGRATORs on almost any controlled parameter. No wonder, cells are so complicated that any additional information can only be beneficial.
LAMBDA does not provide OD or biomass probes for the reasons outlined previously. However, the user can add such probes on its own at a later point. Their output can be connected to the channel “X” or, alternatively, it can be visualized by the SIAM software. The MINIFOR vessel has 6-8 side necks and thus, there is room to put additional probes. However, it should be kept in mind that such measurement methods (the probes and the corresponding controllers) are usually very expensive.