Biyoreaktör - Fermentör
Biyoreaktör - Fermentör
Yenilikler sayesinde düşük maliyetli, yüksek kaliteli bir fermenter üretilmiştir. LAMBDA MINIFOR, fermantasyon ve hücre kültürleri alanında yeni kavramlar geliştirmiştir:
LAMBDA MINIFOR tezgah üstü fermentör ve biyoreaktör
LAMBDA MINIFOR tezgah üstü laboratuar fermantörü ve biyoreaktörü, 35 ml’den 6 litreye kadar hacimlerde küçük bir laboratuar fermentörü inşa etme ihtiyacının bir sonucu olarak geliştirildi. Fermentasyonların uzun kişisel pratik deneyimlerine dayanarak, kullanımı kolay ve biyolojik kültürün bütün önemli parametrelerini ölçme ve kontrol etme kapasitesine sahip bir fermentör yaratmak istedik.
Fermantasyon tezgahı üzerinde minimum yer tutmak zorunda kaldı, ancak tüm parçalara iyi erişimi vardı. Birkaç fermantör, yan yana yerleştirildiğinde, kültür büyüme parametrelerinin optimizasyonu veya biyo-dönüşümlerin optimizasyonu için uygun olmalıdır.
Her fermentör, gelişmiş düzenleme ve kapsamlı veri işlemi için bağımsız olarak çalışabilir veya bir PC’ye bağlanabilir olmalıdır.
- “Kolay sterilite” kavramı
- Sıcaklık, pH, pO2 (çözünmüş oksijen (DO)), hava debisi parametrelerinin otomatik kontrolü
- Tamamı camdan imal edilen, ağızları ve kapakları vidalı yeni kaplar kullanım esnekliğini arttırmıştır
- Tek bir cihazda 35 ml ila 6 litre kültür hacimleri
- Kademeli, hassas ve ekonomik kültür ısıtması için yeni kızılötesi radyatör (pahalı su banyolarına ihtiyaç duyulmaz)
- Son derece kompakt, kullanışlı ve her taraftan rahatça ulaşılabilir
- Hücre kültürlerinin yumuşak ve etkili şekilde karıştırılması için yeni “balık kuyruğu” karıştırıcı
- Kesikli, beslemeli kesikli ve kesintisiz kültür işlemi
- Hassas kütle akış ölçümü ile gaz akış kontrolü
- Otomatik köpürme önleme kontrolü (opsiyonel)
- Çok hızlı ve kolay kurma ve sökme
- Yaygın şekilde kullanılan otoklavlarda sterile edilebilme
- Kullanılan modern yüksek teknoloji ürünü malzemeler
- Tek başına veya bilgisayarla kontrol edilebilme
- FNet veya SIAM fermenter kontrol yazılımı (opsiyonel)
- Paralel fermantasyon prosesleri için de uygundur
- Garanti: 2 yıl
MINIFOR fermantör-biyoreaktörün maliyetini kaliteden ödün vermeden düşük tutmak için birkaç yeni fikir ve yenilik getirildi:
Paslanmaz çelik kapaklı, fermentörlü bir şişe yerine pahalıdır, dişli armatürlerle tüm cam kapları kullanırız. Hücre kültüründe uzun yıllar kullanılmaktadırlar ve kusursuz sterilliği sürdürdüğü kanıtlanmıştır. Bu konsept sayesinde MINIFOR fermantör-biyoreaktör mümkün olan en kısa sürede kurulur.
Pahalı bir motor ve manyetik kuplaj gerektiren geleneksel bir pervane karıştırıcı yerine, yeni bir yukarı-aşağı karıştırma işlemi başlattık. Ucuz bir membranla birlikte bir motor, sterilliği mükemmel bir şekilde garanti eder ve bir vorteks oluşmadan verimli bir şekilde karıştırma sağlar (hiçbir bölme gerekmez). Aynı zamanda bu tür karıştırma hücreler üzerinde daha naziktir ve daha az köpük üretir. Yeni biomimikasyon “balık kuyruğu” karıştırma diskleri, kenarları kesmeden maksimum karıştırma etkinliği sunar.
Kültür, parabolik bir radyatörde üretilen ısı radyasyonu ile fermantasyon kabının altına yerleştirilen altın reflektör ile ısıtılır. Isı, güneş ısıtma suyuna benzer şekilde kültüre hafifçe emilir. Herhangi bir hacimde kültürün aşırı ısınması yoktur ve termostatik banyolar içeren pahalı çift duvarlı kaplar elimine edilir. Aynı zamanda boru ve kablolar yok olur ve fermentörü daha az karmaşık hale getirir.
Mümkün olduğunca pahallı ekipman parçaları yeni yüksek performanslı plastiklerle değiştirildi.
Modern mikroişlemcileri kullanarak tüm elektronikleri cihazın ön kısmına yerleştirmek mümkündür. Bu, fermentörü inanılmaz derecede kompakt yapar ve muhafaza kulesini ortadan kaldırır. Küçük boyutuna rağmen, MINIFOR’un temel konfigürasyonunda altı parametre ölçülmekte ve kontrol edilmektedir.
Dimensions: 22 x 40 x 38 cm (W x D x H)
Display: LCD 4 x 40 digits with backlight illumination
Fermentor vessel: Pyrex glass with 6 to 8 side necks; 0.3, 0.4, 1, 3, 7 liter vessels
Temperature control: High efficiency 150 W infrared (IR) radiation heat source with gilded parabolic reflector
Regulation: from 5°C over RT to 70°C
Measurement: from 0 to 99.9°C in 0.1°C steps
Precision: +/- 0.2°C (0 to 60°C)
Sensor: Pt 100 incorporated in the glass electrode of the pH probe
pH control: sterilisable pH electrode pH 0-14 with automatic temperature correction, two-point semiautomatic calibration and Variopin connector
Resolution: 0.01 pH unit
Precision: +/- 0.02 pH unit
pO2 control: sterilisable Clark type oxygen sensor with fast response, automatic temperature correction, two-point semiautomatic calibration, dissolved oxygen (DO) control through regulation of the airflow rate
Range: 0 to 25 mg oxygen/ l, in 0.1 mg/l steps
Air flow: 0 to 5 l/min in 0.01 l/min steps, measured by precise mass flow meter, linearity +/- 3%, reproducibility +/- 0.5%
Control: proportional valve controlled by microprocessor
For supplied air pressure: 0.05 – 0.2 MPa (0.5 - 2 atm)
Agitation: 50 W Vibromixer 0 to 20 Hz (0 to 1200 rpm) in 0.1 Hz steps (6 rpm) with 1 or more stirring discs; Sterility similar to magnetic coupling
Selectable parameter 'X': an additional parameter can be controlled by the instrument (foaming control, weight (for continuous cultures), pCO2, redox potential, conductivity, optical density, etc.); with standard 0-10V or 0-20mA output
Ports / side necks: One large quadruple sampling or additions port with four needles with LAMBDA PEEK double-seal connections, used for sampling, inoculation, antifoam, feeds, harvest, addition of correction solutions etc., additional double ports are available.
Pumps: up to 4 independent pumps (PRECIFLOW, MULTIFLOW, HIFLOW or MAXIFLOW) with speed variation from 0 to 100 % can be used with MINIFOR lab fermenter-bioreactor
Gas flow control: In addition to pumps, several electronic flow controllers with flow rate ranges of 0-5 l/min (MASSFLOW 5000) or 0-500 ml/min (MASSFLOW 500) can be used for the controlled addition of gases (e.g. N2, O2, air, CO2) in cell cultures; freely configurable gas station module
Working temperature: 0 – 40 °C
Working humidity: 0 - 90 % RH, not condensing
Weight: 7.5 kg
PC control: complete PC control and data processing using the fermentation software FNet (for up to 6 MINIFOR fermenters) or SIAM (for an even higher number of instruments)
- EN - LAMBDA MINIFOR Laboratory Fermenter-Bioreactor LEAFLET (pdf)
- EN - 20 innovations of LAMBDA MINIFOR laboratory-scale bioreactors — Overview and Benefits (pdf)
- EN - LAMBDA MINIFOR laboratory fermenter and bench-top bioreactor at universities and highschools (pdf)
- EN - Operation manual: MINIFOR lab fermentor-bioreactor (pdf)
- EN - Infographics: Overview of innovations in MINIFOR lab fermenter and bioreactor (pdf)
- EN - Quick overview of MINIFOR bioreactor vessels with connections, probes & tubing lines (pdf)
- EN - Peltier electronic cooling loop for Minifor fermentor (pdf)
- EN - PUMP-FLOW INTEGRATOR replaces the need for optical density measurements in fermenters and bioreactors (pdf)
- EN - REDOX potential measurement for MINIFOR laboratory fermentor (pdf)
- EN - MINI-4-GAS, the automatic gas mixing module (pdf)
- EN - How to compare the cost and real value of a laboratory fermentor? (pdf)
- EN - Solitary teamplayer in parallel processes - LAMBDA MINIFOR laboratory fermenter and bioreactor (pdf)
- DE - UNI _ LAMBDA MINIFOR Laborfermenter und Tischbioreaktor an Hochschulen (pdf)
- DE - Montageanleitung _ Kurzübersicht der Bioreaktorgefässe LAMBDA MINIFOR mit Anschlüssen, Sonden und Schlauchlinien (pdf)
- DE - Broschüre _ MINIFOR Laborfermenter Bioreaktor (pdf)
- DE - Parallelreaktor _ LAMBDA MINIFOR Laborfermenter Bioreaktor der Einzelgänger im Parallelsystem (pdf)
- DE - Bedienungsanleitung _ LAMBDA MINIFOR Labor-Tischfermenter und Bioreaktor (pdf)
- DE - LAMBDA MINIFOR Laborfermenter und Bioreaktor - Innovationsuebersicht und Vorteile (pdf)
- FR - MINIFOR - fermenteur de laboratoire (pdf)
- FR - Installation de cuve LAMBDA MINIFOR (pdf)
- FR - LAMBDA MINIFOR Start-Up Kit - mode d'emploie (pdf)
- FR - LAMBDA MINIFOR fermenteur / bioreacteur - Resume des innovations et avantages (pdf)
- FR - LAMBDA MINIFOR fermenteur de laboratoire-bioréacteur de paillasse et son utilisation dans les écoles et universités (pdf)
- FR - Le fermenteur et bioréacteur de laboratoire MINIFOR: Le joueur solitaire dans l’équipe des procédés en parallèle (pdf)
- FR - Comment comparer le coût d’un fermenteur ou bioréacteur avec sa valeur réelle ? (pdf)
- RU - Лабораторный ферменторбиореактор ЛАМБДА МИНИФОР (pdf)
- CZ - MINIFOR - laboratorní fermentor (pdf)
- ES - Manual de operaciones - LAMBDA MINIFOR Fermentador-Biorreactor de Laboratorio (pdf)
- ES - LAMBDA MINIFOR Biorreactor-fermentador de laboratorio - resumen de innovaciones y ventajas (pdf)
- ES - Fermentador-biorreactor de laboratorio LAMBDA MINIFOR para las escuelas y universidades (pdf)
- ES - MINIFOR - fermentador de laboratorio (pdf)
- ES - Fermentador y biorreactor LAMBDA MINIFOR: El solitario en procesos paralelos (pdf)
- ES - ¿Cómo comparar el coste y el valor real de un fermentador y biorreactor de laboratorio? (pdf)
The mouse hybridoma producing the CB.Hep-1 monoclonal antibody (mAb) was cultivated for 100 days in protein-free medium using a Lambda MINIFOR bioreactor. The Lambda MINIFOR bioreactor robustness is adequate for long-term production of mammalian cells at bench-scale. The work was aimed to demonstrate the stability of CB.Hep-1 hybridoma cells cultivated in the Lambda MINIFOR bioreactor operated under continuous mode and supplemented with PFM, for 100 days..
González M, Aragón H, Medel M, Hernández D, Ferro W, Llamo A, et al. Continuous culture of mouse hybridoma cells for 100 Days in the Lambda MINIFOR bioreactor. Biotecnol Apl. 2024;41(2):2211-8.
2025
An in vitro dynamic biofilm model was established using a Lambda Minifor bioreactor (LAMBDA Laboratory Instruments, Baar, Switzerland). The system utilized a sterile modified BHI medium, delivered via a peristaltic pump at constant pressure, to maintain bacterial inoculum under controlled oral-like conditions (37 °C, pH 7.2, anaerobic).
Nuevo, P., Virto, L., Ribeiro-Vidal, H., Gil, J., & Sanz, M. (2025). In vitro assessment of a novel piranha-passivated dental implant surface against oral biofilm formation. Clinical Oral Implants Research, 1–11. https://doi.org/10.1111/clr.70031
Two Lambda Minifor fermenters (1 L, LAMBDA Instruments GmbH, Baar, Switzerland) were used in continuous MCF. Equipped with pH-temperature electrodes, an IR heater, a weighing module, and peristaltic pumps for base, acid, feed, and effluent, the fermenters were maintained at 30 °C with a 4-day HRT. The weighing module controlled effluent to keep constant volume.
Brodowski, F., Gutowska, N., Duber, A., Zagrodnik, R., Łężyk, M., & Oleskowicz-Popiel, P. (2025). Microbial and metabolic variations in pH-dependent chain elongation: Co-utilization of lactate and ethanol vs. lactate-based production. Chemical Engineering Journal, 519, 165367. https://doi.org/10.1016/j.cej.2025.165367
The fermentations were carried out in a 3 L biofermentor (LAMBDA MINIFOR, LAMBDA Laboratory Instruments, Baar, Switzerland) with a 2 L working volume, and samples were taken every 8 h. Optical density, sugar concentration, and lactic acid content were monitored.
Mabia, A. G., Andrianisa, H. A., Danielli, C., Ouattara, L. Y., Kouadio, N. E., Kouassi, E. K. A., Gardossi, L., & Yao, K. B. (2025). Exploring the Synthesis of Lactic Acid from Sugarcane Molasses Collected in Côte d’Ivoire Using Limosilactobacillus fermentum ATCC 9338 in a Batch Fermentation Process. Bioengineering, 12(8), 817. https://doi.org/10.3390/bioengineering12080817
Co-culturing & Anaerobic fermentation: The mixed inoculum was introduced into the reaction chamber of the Lambda Minifor bioreactor (LAMBDA Laboratory Instruments, Sihlbruggstrasse, Switzerland), which was maintained at 37°C under anaerobic conditions. Planktonic growth within the bioreactor was allowed for 12 hours, after which the culture was maintained in continuous operation with a flow rate of 30 mL/h using a peristaltic pump..
Khan, S. N., Ribeiro-Vidal, H., Virto, L., Bravo, E., Nuevo, P., Koldsland, O. C., Hjortsjö, C., & Sanz, M. (2025). The decontamination effect of an oscillating chitosan brush compared with an ultrasonic PEEK-tip: An in vitro study using a dynamic biofilm model. Clinical Oral Implants Research, 36(1), 73–81. https://doi.org/10.1111/clr.14360
Biocatalytic research: The study focuses on optimizing the second step of a biocatalytic process for producing 2-GLVs using soybean 13-lipoxygenase in a controlled laboratory fermenter (Lambda Minifor), highlighting parameters such as temperature, reaction time, oxygen flow, enzyme load, and substrate concentration.
Faillace, E., Brunini-Bronzini de Caraffa, V., Gambotti, C., Berti, L., Maury, J., & Vincenti, S. (2025). Optimizing the second step of the biocatalytic process for green leaf volatiles production: Synthesis by soybean 13-lipoxygenase of 13-hydroperoxides from hydrolyzed hempseed oil. Biocatalysis and Agricultural Biotechnology, 65, Article 103548. https://doi.org/10.1016/j.bcab.2025.103548
LAMBDA MINIFOR bioreactor was used to perform submerged fermentation of cocoa pod husks (Theobroma cacao L.) for lactic acid production using Lactobacillus fermentum ATCC 9338.
The system enabled precise control of pH, temperature, agitation, and aeration throughout the fermentation process.
Ouattara, L. Y., Konan, A. T. S., Mahamane Nassirou, A. K., Sanou, A., Mabia, A. G., Appiah Kouassi, E. K., Soro, D., Yao, K. B., Drogui, A. P., & Tyagi, D. R. (2025). Use of cocoa pods husks (Theobroma cacao L.) as a potential substrate for the production of lactic acid by submerged fermentation using Lactobacillus fermentum ATCC 9338. Waste and Biomass Valorization. https://doi.org/10.1007/s12649-025-03010-y
Small-scale batch fermentations of L. coryniformis: We used a Lambda Minifor bioreactor system with 35–400-mL working capacity vessels. The working volume was 300 mL. The fermentations were maintained at 30°C, stirring 0.5 Hz, CO2 flow of 0.04 L/min, and pH 6.5 for 72 h... Bioreactor fermentations were conducted in five independent replicates F1–F5.
Elvan Gezer M, Gravlund Fønss K, Bambace MF, Marietou A, Sandberg Overby S, Sundekilde U, Schwab C.2025.Investigation on L-rhamnose metabolism of Loigolactobacillus coryniformis subsp. coryniformis DSM 20001 and its propionate-containing fermentates. Appl Environ Microbiol91:e01613-24. https://doi.org/10.1128/aem.01613-24
2024
Simultaneous Saccharification and Fermentation (SSF): The LAMBDA MINIFOR bioreactor was used for both the fermentation of pretreated solid biomass (10 %) in the presence of cellulase, β-glucosidase and inoculated with Lactobacillus rhamnosus (1.7 L, 37 °C, pH=5.5, 72 h), as well as for the subsequent fermentation of hydrolysates with Bacillus megaterium (1.7 L, 35 °C, 48 h).
Senila, L., Kovacs, E., Resz, M. A., Senila, M., Becze, A., & Roman, C. (2024). Life Cycle Assessment (LCA) of Bioplastics Production from Lignocellulosic Waste (Study Case: PLA and PHB). Polymers 2024, 16, 3330.
https://doi.org/10.3390/polym16233330
Anaerobic batch cultivation of C. butyricum in a LAMBDA MINIFOR 3L fermenter, flushed with argon prior to fermentation: pH 5.6 (controlled with 1M NaOH), 37°C and 6000 rpm. Gas production was measured by the water displacement method, displaced water volume monitored by a camera. Liquid and gas samples were collected at intervals.
Pištěková, H., Dusankova, M., Sopik, T., Klaban, J., Dostalkova, J., Moučka, R., & Sedlarik, V. Optimization of the Dark Fermentation Technique for Hydrogen Production Through Supplementation with Ascorbic Acid And/Or L-Cysteine by Clostridium Butyricum Ccdbc 11. Or L-Cysteine by Clostridium Butyricum Ccdbc, 11.
https://dx.doi.org/10.2139/ssrn.4973469
Alcoholic fermentations tested under microvinification conditions and in a LAMBDA MINIFOR bioreactor with controlled parameters (T = 20 °C, agitation frequency = 2 Hz) and standard conditions (T = 12 - 14 °C).
Filimon, G. (2024). Monitorizarea fermentaţiei alcoolice a musturilor obţinute din soiuri de struguri rizogene. Universitatea Tehnică a Moldovei. Technical Scientific Conference of Undergraduate, Master and PhD Students: Chişinău, 27-29 martie 2024. Vol. 2.
URI: http://repository.utm.md/handle/5014/28059
Biodegradation: Treatment of marine samples with the addition of “Diesel-B5” (10 %, 15 %, 20 %) as a C-source in aerated and stirred LAMBDA MINIFOR bioreactors (Pseudomonas sp.; pH 6.5; incubation temperatures 25 °C, 35 °C and 45 °C).
Peña Ventura, J. C. (2024). Determinación del ratio de biodegradación de hidrocarburos totales de petróleo empleando Pseudomonas sp. en agua de mar contaminada con diésel-B5. Universidad Nacional de Trujillo, Unidad de Postgrado en Ingeniería Química, Tesis de maestro en ingeniería química ambiental.
URI: https://hdl.handle.net/20.500.14414/22166
"The mixed inoculum was inoculated into the reaction chamber of the LAMBDA MINIFOR bioreactor, which was maintained at 37 °C under anaerobic conditions."
Khan, S. N., Ribeiro‐Vidal, H., Virto, L., Bravo, E., Nuevo, P., Koldsland, O. C., Hjortsjö, C. & Sanz, M. (2024). The Decontamination Effect of an Oscillating Chitosan Brush Compared With an Ultrasonic PEEK‐Tip: An In Vitro Study Using a Dynamic Biofilm Model. Clinical Oral Implants Research.
https://doi.org/10.1111/clr.14360
The batch co-culture fermentation of date palm syrup with S. cerevisiae and P. stipitis ATCC 58785 was carried out in a LAMBDA MINIFOR 1L Advanced Kit stirred tank fermenter.
Shalsh, F. J., Gatte, E. H., Alsultan, I. I., Tariq, F. Z., Jassim, S. M., Saleh, M. T., & Alrazzaq, N. A. (2024). Bioplastic Compounds of Succinic Acid from Agriculture Waste; Date Palm Syrup And Date Palm Fronds. Nepal Journal of Biotechnology, 12(1), 32-39.
https://doi.org/10.54796/njb.v12i1.310
In vitro development of a dynamic multispecies biofilm model on implant surfaces: Planktonic growth of a mixture of six different bacterial strains inside the LAMBDA MINIFOR bioreactor (12 h), followed by continuous culture (flow rate of 30 mL/h) through 2 Robbins devices containing the sterile implants (37 °C, anaerobic, 72 h).
Khan, S. (2024). Efficacy of Mechanical Decontamination Strategies in the Treatment of Peri-implantitis. Doctoral thesis, University of Oslo.
URI: https://hdl.handle.net/10852/111365 (2024 August 07)
In vitro dynamic multispecies biofilm model: During the entire incubation process (biofilms growing on implant surfaces), the LAMBDA MINIFOR bioreactor maintains the culture medium at 37 °C, pH 7.2 and an anaerobic atmosphere (10% H2, 10% CO2 and N2 balance).
Bravo, E., Arce, M., Ribeiro-Vidal, H., Herrera, D., & Sanz, M. (2024). The Impact of Candida albicans in the Development, Kinetics, Structure, and Cell Viability of Biofilms on Implant Surfaces—An In Vitro Study with a Validated Multispecies Biofilm Model. International Journal of Molecular Sciences, 25(6), 3277.
https://doi.org/10.3390/ijms25063277
Submerged batch (consortia) and fed-batch (L. coryniformis) fermentations in LAMBDA MINIFOR stirred-tank bioreactor and SIAM software: 72 h, final working volumes = 200 ml, T = 37 ± 0.5 °C, stirring 0.5 Hz, sparing CO2 at 0.04 L/min, pH 6.5 ± 0.5 using 1 M NaOH and 1 M H2SO4 for automatic pH adjustment; samples were taken in regular intervals.
Buljubašić, E., Bambace, M. F., Christensen, M. H. L., Ng, K. S., Huertas‐Díaz, L., Sundekilde, U., Marietou, A. & Schwab, C. (2024). Novel Lactobacillaceae strains and consortia to produce propionate‐containing fermentates as biopreservatives. Microbial Biotechnology, 17(2), e14392.
https://doi.org/10.1111/1751-7915.14392
The in vitro multispecies dynamic biofilm model was used which has been validated on biofilms growing on implant surfaces: LAMBDA MINIFOR bioreactor maintains the protein-enriched BHI medium under stable conditions: 37 °C, pH 7.2 and an anaerobic atmosphere by directly pumping an anaerobic gas mixture (10% H2, 10% CO2 and equilibrium N2) keeping the pressure constant during the whole incubation process
Bravo, E., Arce, M., Ribeiro-Vidal, H., Herrera, D., & Sanz, M. (2024). The Impact of Candida albicans in the Development, Kinetics, Structure, and Cell Viability of Biofilms on Implant Surfaces. An In Vitro Study with a Validated Multispecies Biofilm Model.
https://doi.org/10.20944/preprints202402.1137.v1
Simulated human colon model LAMBDA MINIFOR with basal nutrient medium (37 °C, pH 6.8) and faecal slurries
Pusuntisumpun, N., Tunsagool, P., Nitisinprasert, S., & Nakphaichit, M. (2024). Impacts of combining Limosilactobacillus reuteri KUB‐AC5 and Limosilactobacillus fermentum KUB‐D18 on overweight gut microbiota using a simulated human colon model. International Journal of Food Science & Technology.
https://doi.org/10.1111/ijfs.16941
400 ml isolated tomato cells in a LAMBDA MINIFOR bioreactor (20 °C, pH 5.8) got sparged (10 L/h) to achieve varied O2 concentrations (21 kPa, 5 kPa, and 0 kPa) for the study of postharvest losses of fruits and vegetable during controlled atmosphere storage as a result of low O2 stress.
Mahomud, M. S., Islam, M., & Roy, J. (2024). Effect of low oxygen stress on the metabolic responses of tomato fruit cells. Heliyon, e24566.
https://doi.org/10.1016/j.heliyon.2024.e24566
Hülber-Beyer, É. A., Nemestóthy, N., & Bélafi-Bakó, K. (2023). Case Study of Continuous Itaconic Acid Fermentation by Aspergillus Terreus in a Bench-Scale Bioreactor. Hungarian Journal of Industry and Chemistry, 51(2), 57-63.
Pictures of morphology during fermentation: Fig. 1 on https://hjic.mk.uni-pannon.hu/index.php/hjic/article/view/1214/1092 (2024 Feb. 08)
Senila, L., Cadar, O., Kovacs, E., Gal, E., Dan, M., Stupar, Z., Simedru, D., Senila, M. & Roman, C. (2023). L-Poly(lactic acid) Production by Microwave Irradiation of Lactic Acid Obtained from Lignocellulosic Wastes. Int. J. Mol. Sci. 2023, 24, 9817.
Senila, L., Gál, E., Kovacs, E., Cadar, O., Dan, M., Senila, M. & Roman, C. (2023). Poly(3-hydroxybutyrate) Production from Lignocellulosic Wastes Using Bacillus megaterium ATCC 14581. Polymers. 2023, 15, 4488.
Bravo, E., Serrano, B., Ribeiro-Vidal, H., Virto, L., Sánchez, I.S., Herrera, D. & Sanz, M. (2023). Biofilm formation on dental implants with a hybrid surface microtopography: An in vitro study in a validated multispecies dynamic biofilm model. John Wiley & Sons, Ltd., 0905-7161. Clinical Oral Implants Research, Volume 34, Issue 5, May 2023 Pages i-iii, 405-541.
Simulation of in-vitro gut model with LAMBDA MINIFOR 0.3L: Batch fermentations under anaerobic conditions (37 °C, pH 6.8–6.9, 24 h) of human fecal slurry (1% (v/v)) to evaluate the effect of Triphala extracts (1 mg/mL) co-fermentation on microbiota and metabolic changes.
Kwandee, P., Somnuk, S., Wanikorn, B., Nakphaichit, M. & Tunsagool, P. (2023). Efficacy of Triphala extracts on the changes of obese fecal microbiome and metabolome in the human gut model. Journal of Traditional and Complementary Medicine, Volume 13, Issue 2, 2023, Pages 207-217, ISSN 2225-4110.
Gastrointestinal Simulation Model SHIME with LAMBDA MINIFOR 0.3L reactors:
Each Simulator of Human Intestinal Microbial Ecosystem consists of five reactors, mimicking different sections of the human Gastrointestinal tract.
The video https://www.youtube.com/watch?v=hXcpa0bXu6Q shows Assoc. Prof. Massalin Nakphaichit in front of the SHIME reactors.
Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University
In a bench-scale LAMBDA MINIFOR 7L bioreactor with programmable probes, RODMs (a set of organisms with novel metabolism, efficiently degrading highly-concentrated aromatics) were developed for a high-density microbial ecosystem.
Ahmad, M., Yousaf, M., Han, J.-C., Huang, Y., Zhou, Y. & Tang, Z. (2023). Development of Biocatalytic Microbial Ecosystem (FPUS@RODMs@In-PAOREs) for Rapid and Sustainable Degradation of Various Refractory Organics. Journal of Hazardous Materials, 2023, 131514, ISSN 0304-3894,
https://doi.org/10.1016/j.jhazmat.2023.131514
Hybridoma cell (inoculation 4.0×10E5 cells/mL (90% viability)): Fed-batch in a LAMBDA MINIFOR stirred-tank bioreactor.
Llamo, A., Hernández, D., García, C., González, M., Ferro, W., Garay, H., Diago, D., Fajardo, A., Espinosa, L., Padilla, S., Gómez, L., Chinea, G. & and Valdés, R. (2023). Gamma-Immunoglobulin Response Characterization, in COVID-19 Convalescent Patients, Against the Spike Protein S2 Subunit with Eight Linear Peptides for Monoclonal Antibody Generation. BioProcess J, 2023; 22.
https://doi.org/10.12665/J22OA.Llamo
Optimization of lipase-catalyzed hydrolysis of vegetable oils: Control and monitoring by a LAMBDA MINIFOR bioreactor (pH, temperature, reaction time, enzyme load and oil/aqueous ratio of the reaction mixture).Faillace, E., Brunini-Bronzini de Caraffa, V., Mariani, M., Berti, L., Maury, J. & Vincenti, S. (2023). Optimizing the First Step of the Biocatalytic Process for Green Leaf Volatiles Production: Lipase-Catalyzed Hydrolysis of Three Vegetable Oils. International Journal of Molecular Sciences. 2023; 24(15):12274.
https://doi.org/10.3390/ijms241512274
Evaluation of viability and maximum specific growth rate of Bacillus licheniformis in a bench-top LAMBDA MINIFOR 7L laboratory bioreactor (2 litres working volume, 37 °C, pH 6.5, 200 rpm, controlled oxygen, submerged batch fermentation (SMF), addition of sterilized Antifoam 204 agent (Sigma-Aldrich))
Dumitru, M. & Ciurescu, G. (2023). Optimization of the fermentation conditions and survival of Bacillus licheniformis as freeze-dried powder for animal probiotic applications. Scientific Papers. Series D. Animal Science. Vol. LXVI, No. 2, 2023; ISSN 2285-5750; ISSN CD-ROM 2285-5769; ISSN Online 2393-2260; ISSN-L 2285-5750. https://www.animalsciencejournal.usamv.ro/pdf/2023/issue_2/Art10.pdf (2024 Jan. 02)
Alonso-Español, A., Bravo, E., Ribeiro-Vidal, H., Virto, L., Herrera, D., Alonso, B. & Sanz, M. (2023). The Antimicrobial Activity of Curcumin and Xanthohumol on Bacterial Biofilms Developed over Dental Implant Surfaces. Int. J. Mol. Sci. 2023, 24, 2335.
Milk was pasteurized at 70 °C for 30 min in LAMBDA MINIFOR fermenters
LAMBDA MINIFOR fermenters: Temperature effect tests on growth of yeast Kazachstania unispora (initial ~10E6 CFU/ml) in milk (800 ml, ~6 % lactose): from 5 °C to 40 °C (5, 10, 15, 20, 25, 27, 30, 32, 35, 37, and 40 °C) at pH 5.6 (automatic adjustment with 2 M NaOH) and 240 rpm, until stationary phase was reached (inline near-infrared turbidity sensor Optek FC20- ASD10-N)
LAMBDA MINIFOR fermenters for coculture experiments at 25 °C of Lacticaseibacillus casei and Kazachstania unispora in modfied MRS media as well as in mare milk (initial: ~10E6 CFU/ml, pH = 6.8)
LAMBDA MINIFOR fermenters for coculture experiments at 30 °C of Lactobacillus kefiri and Kazachstania unispora in modfied MRS media as well as in mare milk (initial: ~10E6 CFU/ml, pH = 6.8)
Kondybayev, A., Achir, N., Mestres, C., Collombel, I., Strub, C., Grabulos, J., Akhmetsadykov, N., Aubakirova, A., Kamidinkyzy, U., Ghanmi, W. & Konuspayeva, G. (2023). Growth Kinetics of Kazachstania unispora and Its Interaction with Lactic Acid Bacteria during Qymyz Production. Fermentation 2023, 9, 101.
https://doi.org/10.3390/fermentation9020101
Pasotti, L., De Marchi, D., Casanova, M., Frusteri Chiacchiera, A., Cusella De Angelis, M. G., Calvio, C., & Magni, P. (2023). Design of a stable ethanologenic bacterial strain without heterologous plasmids and antibiotic resistance genes for efficient ethanol production from concentrated dairy waste. Biotechnology for Biofuels and Bioproducts, 16(1), 1-13.
https://doi.org/10.1186/s12896-017-0369-y
2022:
www.geniusjournals.org/index.php/emrp/article/view/2162 (2022 Sept. 22)
LAMBDA MINIFOR bioreactor and fermentor for schooling & university
Lactic acid bacteria growth experiments: Effect of temperature on Lacticaseibacillus casei and Lactobacillus kefiri.
Kondybayev, A.; Konuspayeva, G.; Strub, C.; Loiseau, G.; Mestres, C.; Grabulos, J.; Manzano, M.; Akhmetsadykova, S. & Achir, N. (2022). Growth and Metabolism of Lacticaseibacillus casei and Lactobacillus kefiri Isolated from Qymyz, a Traditional Fermented Central Asian Beverage. Fermentation 2022, 8, 367.
During 65 days, two continuous (HRT= 5 days) stirrer tank fermenters LAMBDA MINIFOR were operated under anaerobic conditions (N2 into headspace & sparging), each with 1 liter working volume (modification of lactate / acetate concentrations) inoculated with caproate-producing sludge (Caproiciproducens genus (Ruminococcaceae family)), temperature control (30 °C, build-in IR heater, Mettler InPro 3253 probe) and pH control (pH 5.5, NaOH 2M, HCl 0.5M) ) with four peristaltic pumps (feed, effluent, base & acid) and daily liquid sampling for carboxylates and alcohols analysis.
Brodowski, F., Lezyk, M., Gutowska, N., Kabasakal, T. & Oleskowicz-Popiel, P. (2022). Influence of lactate to acetate ratio on biological production of medium chain carboxylates via open culture fermentation. Science of The Total Environment, Volume 851, Part 1, 2022, 158171, ISSN 0048-9697.
The continuous culture was performed in a Lambda Photobioreactor (PBR). White light from the LAMBDA LUMO module was calibrated to umolm−2 m−1. For evaporation control and continuous culture mode, the total weight of the reactor setup was kept constant using the built-in LAMBDA reactor mass control module and automatic addition of fresh culture medium through the feed pump. Continuous culture was performed by setting the waste pump to a fixed speed.
Behle, A., Dietsch, M., Goldschmidt, L., Murugathas, W., Berwanger, L.C., Burmester, J., Yao, L., Brandt, D., Busche, T., Kalinowski, J., Hudson, E.P., Ebenhöh, O., Axmann, I.M. & Machné, R. (2022). Manipulation of topoisomerase expression inhibits cell division but not growth and reveals a distinctive promoter structure in Synechocystis. Nucleic Acids Research, Volume 50, Issue 22, 9 December 2022, Pages 12790–12808.
https://doi.org/10.1093/nar/gkac1132
Biocatalytic resolution of lupanine racemate in industrial wastewater by Pseudomonas putida LPK411 using a lab‐scale bioreactor LAMBDA MINIFOR 0.4L under batch operation.
Parmaki, S., Esteves, T., Gonçalves, J.M.J. Catenacci, A., Malpei, F., Ferreira, F.C., Afonso C.A.M & Koutinas, M. (2022). Selective microbial resolution of lupanine racemate: Bioprocess development and the impact of carbon catabolite repression on industrial wastewater valorisation. Biomass Conv. Bioref. (2022).
https://doi.org/10.1007/s13399-022-03383-3
For releasing bioactive peptides, diluted Kiwicha protein isolate (KPI) from Amaranthus caudatus L. seed flour was subjected to enzymatic hydrolysis with Bioprotease LA-660 using a LAMBDA MINIFOR bioreactor for continuous stirring, pH- and temperature control (pH 8; 50 °C).
Martinez-Lopez, A., Rivero-Pino, F., Villanueva, A., Toscano, R., Grao-Cruces, E., Marquez-Paradas, E., Martin, M.E., Montserrat-de la Paz, S. & Millan-Linaresa, M.C. (2022). Kiwicha (Amaranthus caudatus L.) protein hydrolysates reduce intestinal inflammation by modulating the NLRP3 inflammasome pathway. Food & Function 2022 Oct 21.
https://doi.org/10.1039/D2FO02177C
Escherichia coli (E. coli; E44Δ) mutant strain for production of large quantity of outer membrane vesicles (OMVs) in a LAMBDA MINIFOR 7L fermenter.
Allahghadry, T., Bojesen, A.M., Whitehead, B.J. and Antenucci, F. (2022). Clarification of large-volume bacterial cultures using a centrifuge-free protocol. J Appl Microbiol. Accepted Author Manuscript.
https://doi.org/10.1111/jam.15608
2021:
Experiments on liquid phase (hemicelluloses hydrolysate) for xylitol production: The fermentation of 250 ml of detoxified hydrolysate was conducted in a 1L fermenter (LAMBDA MINIFOR bench-top-laboratory-fermenter) and pH adjustment (pH 5.0) at aerobic conditions at 30 °C for 60 hr.
Shalsh, D., Nagimm, D., Alrheem, M.A. & Alrheem, S.A. (2021). Batch fermentation and Simultaneous Saccharification and Fermentation (SSF) processes by Meyerozyma Guilliermondii Strain F22 and Saccharomyces cerecvisae for xylitol and bioethanol co-production. Al-Qadisiyah Journal of Pure Science, 26(4), 80–94.
https://doi.org/10.29350/qjps.2021.26.4.1347
The growth, glucose consumption and ethanol production of Saccharomyces cerevisiae LM strain in synthetic broth were modeled for the most important intrinsic...One liter Lambda Minifor fermenters equipped with a cold water condenser on air exit pipes (LAMBDA Instruments GmbH, Baar- Switzerland), were used.
Kouamé, C., Loiseau, G., Grabulos, J., Boulanger, R. & Mestres, C. (2021). Development of a model for the alcoholic fermentation of cocoa beans by a Saccharomyces cerevisiae strain. International Journal of Food Microbiology, Volume 337, 2021, 108917, ISSN 0168-1605.
https://doi.org/10.1016/j.ijfoodmicro.2020.108917
Continuous culture of cyanobacteria Synechocystis sp. PCC 6803 in a LAMBDA MINIFOR 1L PBR photo-bioreactor.
Behle, A., Dietsch, M., Goldschmidt, L., Murugathas, W., Brandt, D., Busche, T., Kalinowski, J., Ebenhöh, O., Axmann, I. M. & Machné, R. (2021) Uncoupling of the Diurnal Growth Program by Artificial Genome Relaxation in Synechocystis sp. PCC 6803. bioRxiv 2021.07.26.453758.
https://doi.org/10.1101/2021.07.26.453758
The hydrolysis of kiwicha protein isolate (KPI) is performed under continuous stirring, using a LAMBDA MINIFOR fermenter-bioreactor, at controlled conditions of pH and temperature: Bioprotease LA-660 is added at a ratio enzyme/substrate = 0.3 AU/g protein (pH 8) for 5, 10, 15, 30, and 60 min.
Paz, S. M. D. L., Martinez-Lopez, A., Villanueva-Lazo, A., Pedroche, J., Millan, F., & Millan-Linares, M. C. (2021). Identification and characterization of novel antioxidant protein hydrolysates from kiwicha (Amaranthus caudatus L.). Antioxidants, 10(5), 645.
https://doi.org/10.3390/antiox10050645
The biological transformation of white sorghum biomass was performed under operating conditions similar to the MixAlco process. MixAlco batch fermentation process were performed in the LAMBDA MINIFOR bioreactor.
Shalsh, F.J., Alrazzaq, N.A., Nagimm D.K., Alrheem, M.A., Alrheem S.A. & Abd-alalah, K. (2021). Bioconversion of white sorghum biomass using MixAlco fermentation process. DYSONA – Applied Science. 2021(2), 21-27. ISSN 2708-6283.
https://doi.org/10.30493/DAS.2021.248966
2020:
Different yeast strains were cultivated in the LAMBDA MINIFOR 0.4L Fermentor to study the metabolic cylce and pathway.
J. Feltham, S. Xi, S. Murray, M. Wouters, J. Urdiain-Arraiza, C. George, A. Townley, E. Roberts, R. Fisher, S. Liberatori, S. Mohammed, B. Kessler & J. Mellor. (2020). Transcriptional changes are regulated by metabolic pathway dynamics but decoupled from protein levels. bioRxiv 833921.
https://doi.org/10.1101/833921
LAMBDA MINIFOR Bioreactor is used as a Rumen membrane bioreactor to produce volatile fatty acids (VFA) from crop residues (lignocellulosic biomass) by mimicking the digestive system of ruminant animals.
Nguyen, A.Q., Nguyen, L.N., Abu Hasan Johir, M., Ngo, H-H., Chaves, A.V. & Nghiem, L.D. (2020) Derivation of volatile fatty acid from crop residues digestion using a rumen membrane bioreactor: a feasibility study. Bioresource Technology 2020.
https://doi.org/10.1016/j.biortech.2020.123571
Enzymatic hydrolysis experiments were carried out in the lab-scale LAMBDA MINIFOR stirred-batch bioreactor. The pretreated vine-shoot waste was delignified with sodium chlorite for lignin removal and then enzymatically hydrolyzed using new types of enzymes (cellulase from Trichoderma reesei and b-glucosidase).
Eniko Kovacs, Daniela Alexandra Scurtu, Lacrimioara Senila, Oana Cadar, Diana Elena Dumitras & Cecilia Roman (2020). Green Protocols for the Isolation of Carbohydrates from Vineyard Vine-Shoot Waste. Analytical Letters.
https://doi.org/10.1080/00032719.2020.1721001
LAMBDA MINIFOR bioreactor used to produce Itaconic Acid biotechnologically by Aspergillus terreus fungal strain from glucose
Nemestóthy, N., Komáromy, P., Bakonyi, P. et al. (2020). Carbohydrate to Itaconic Acid Conversion by Aspergillus terreus and the Evaluation of Process Monitoring Based on the Measurement of CO2 Waste and Biomass. Valorization 2020.
https://doi.org/10.1007/s12649-019-00729-3
2019:
LAMBDA MINIFOR bioreactor used in turbidostat experiments with recombinant cells in continuous culture operation mode
Pasotti, L., Bellato, M., Politi, N., Casanova, M., ucca, S., Gabriella, M., De Angelis, C. & Magni, P. (2019). A synthetic close-loop controller circuit for the regulation of an extracellular molecule by engineered bacteria. IEEE Trans Biomed Circuits Syst. 2019 Feb; 13(1):248-258.
https://doi.org/10.1109/TBCAS.2018.2883350
pH optimization for the aerobical production of itaconic acid catalyzed by Aspergillus terreus in a LAMBDA MINIFOR bioreactor: Batch, working volume 1.8 L, medium with 120 g/L glucose substrate, 37 °C, pH 3 - pH 2.5 - pH 4 - pH 3 - pH 2.5, stirrer 2 Hz, aeration 1.5 L (STP)/min.
Komáromy, K., Bakonyi, P., Kucska, A., Tóth, G., Gubicza, L., Bélafi-Bakó, K. & Nemestóthy, N. (2019). Optimized pH and Its Control Strategy Lead to Enhanced Itaconic Acid Fermentation by Aspergillus terreus on Glucose Substrate. Fermentation 2019, 5(2), 31
https://doi.org/10.3390/fermentation5020031
Lab-Scale Production of Rhamnolipid by Pseudomonas Aeruginosa A3 using LAMBDA MINIFOR Benchtop Bioreactor.
Faqri, A. F., Hayder, N.H. & Hashim, A.J. (2019). Lab-scale production of Rhamnolipid by Pseudomonas Aeruginosa A3 and study its synergistic effect with certain antibiotics against some pathogenic bacteria. Iraqi Journal of Agricultural Sciences –2019:50(5):1290-1301.
https://doi.org/10.13140/RG.2.2.10802.35520
2018:
A large-scale pro-siRNA production method was developed in a LAMBDA MINIFOR bioreactor for high yield production of pro-siRNA
Kaur, G., Cheung, H. C., Xu, W., Wong, J. V., Chan, F. F., Li, Y., McReynolds, L. & Huang, L. (2018). Milligram scale production of potent recombinant small interfering RNAs in Escherichia coli. Biotechnology and Bioengineering, 115(9), 2280-2291.
https://doi.org/10.1002/bit.26740
LAMBDA MINIFOR lab scale bioreactor used for production of bioethanol from lignocellulosic biodegradable municipal solid waste (BMSW) under optimized conditions
Hayder, N. H., Flayeh, H. M., & Ahmed, A. W. (2018). Optimization of bioethanol production from biodegradable municipal solid waste using response surface methodology (RSM). Journal of Engineering and Sustainable Development, 22(1), 47-64.
https://www.iasj.net/iasj/download/28dcbea4ab5f5ba8 (2024 Feb. 05)
2017:
Comparison of the experimental and theoretical production of biogas. The LAMBDA MINIFOR bioreactor filled with 2 litres of inoculum was incubated anaerobically at 35 °C for 1 month.
El Asri, O., & Afilal, M. E. (2018). Comparison of the experimental and theoretical production of biogas by monosaccharides, disaccharides, and amino acids. International Journal of Environmental Science and Technology, 15(9), 1957-1966.
https://doi.org/10.1007/s13762-017-1570-1
Study of the metabolism of isolated lamb’s lettuce cells (Valerianella locusta (L). Laterr.) upon sugar starvation under O2 stress conditions using 13C [U-13C6] labelled glucose in a LAMBDA MINIFOR bench-top laboratory bioreactor (dark, 250 ml, pH 5.8, 18 °C, airation 10 L/h)
Victor, B. M. M., Ampofo-Asiama, J., Hertog, M., Geeraerd, A. H., & Nicolai, B. M. (2017). Metabolic profiling reveals a coordinated response of isolated lamb's (Valerianella locusta, L.) lettuce cells to sugar starvation and low oxygen stress. Postharvest Biology and Technology, 126, 23-33.
https://doi.org/10.1016/j.postharvbio.2016.12.004
LAMBDA MINIFOR fermenters used as continuous anaerobic flow stirred digesters (CSTR) for anaerobic digestion of organic solid waste
Nakasima-López, M., Taboada-González, P., Aguilar-Virgen, Q., & Velázquez-Limón, N. (2017). Adaptación de inóculos durante el arranque de la digestión anaerobia con residuos sólidos orgánicos. Información tecnológica, 28(1), 199-208.
https://dx.doi.org/10.4067/S0718-07642017000100020
The effect of different temperatures on sugar starvation in cells isolated from fresh leafy vegetables was studied in LAMBDA MINIFOR bioreactor
Mbong, V. B. M., Ampofo-Asiama, J., Hertog, M. L., Geeraerd, A. H., & Nicolai, B. M. (2017). The effect of temperature on the metabolic response of lamb’s lettuce (Valerianella locusta,(L), Laterr.) cells to sugar starvation. Postharvest Biology and Technology, 125, 1-12.
https://doi.org/10.1016/j.postharvbio.2016.10.013
LAMBDA MINIFOR bioreactor for the production of CB.Hep-1 mAb using mouse hybridoma cell culture in protein-free media
Valdés, R., Aragón, H., González, M., Hernández, D., Geada, D., Goitizolo, D., Ferro, W., Pérez, A., García, J., Masforrol, Y, Aguilar, P., Márquez, G., LaO, M., González, T., Calvo, Y., Hernández, A., Menéndez, G. & Tamayo, A. (2017). Mouse Hybridoma Cell Culture in a Protein-Free Medium Using a Bio-Mimicking Fish-Tail Disc Stirred Bioreactor. BioProcessing Journal, 16(1).
https://doi.org/10.12665/J161.Valdes
2016:
Robust cellulosic ethanol production from sugarcane bagasse with Saccharomyces cerevisiae ATCC 20602 in LAMBDA MINIFOR laboratory bioreactor under aerobic and anaerobic conditions with controlled redox potential measurement
Jabasingh, S. A., Lalith, D., Prabhu, M. A., Yimam, A., & Zewdu, T. (2016). Catalytic conversion of sugarcane bagasse to cellulosic ethanol: TiO2 coupled nanocellulose as an effective hydrolysis enhancer. Carbohydrate polymers, 136, 700-709.
https://doi.org/10.1016/j.carbpol.2015.09.098
2015:
Bioreactor system LAMBDA MINIFOR as an artificial mouth system for the growth of biofilms
LA BOCA ARTIFICIAL DE DENTAID UNA REVOLUCIÓN EN INVESTIGACIÓN BUCODENTAL
DENTAID EXPERTISE, PUBLICACIÓN PARA PROFESIONALES DE LA ODONTOLOGÍA, NÚM. 18.
https://aprenderly.com/doc/3463742/la-boca-artificial-de-dentaid-una-revolución-en-investiga…?page=5 (2024 Feb. 05)
S. pyogenes Cas9 protein was expressed in E. coli using a computer-controlled LAMBDA MINIFOR 3L bioreactor in batch mode followed by exponential feeding
Ménoret, S., De Cian, A., Tesson, L., Remy, S., Usal, C., Boulé, J. B., Boix, C., Fontanière, S., Crénéguy, A., Nguyen, T.H., Brusselle, L., Thinard, R., Gauguier, D., Concordet, J.-P., Cherifi, Y., Fraichard, A., Giovannangeli, C. & Anegon, I. (2015). Homology-directed repair in rodent zygotes using Cas9 and TALEN engineered proteins. Scientific reports, 5(1), 14410.
https://doi.org/10.1038/srep14410
Fermentation of engineered microorganism in laboratory scale bioreactor LAMBDA MINIFOR for efficient conversion of lactose-to-ethanol
Pasotti, L., Zucca, S., Casanova, M., Massaiu, I., Mazzini, G., Micoli, G., Calvio, C., Cusella de Angelis, M.G. & Magni, P. (2015, August). Methods for genetic optimization of biocatalysts for biofuel production from dairy waste through synthetic biology. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 953-956). IEEE.
https://doi.org/10.1109/EMBC.2015.7318521
Six-species flow cell biofilm model was developed by culturing bacteria in LAMBDA MINIFOR bioreactor to evaluate the biofilm development under flow and shear conditions
Salli, K. M., & Ouwehand, A. C. (2015). The use of in vitro model systems to study dental biofilms associated with caries: a short review. Journal of oral microbiology, 7(1), 26149.
https://doi.org/10.3402/jom.v7.26149
Quantification of ribosomal proteins (RPs) from Yeast cells cultured in LAMBDA MINIFOR bioreactor and mouse embryonic stem cells (ESC) to study the core RPs stoichiometry
Slavov, N., Semrau, S., Airoldi, E., Budnik, B., & van Oudenaarden, A. (2015). Differential stoichiometry among core ribosomal proteins. Cell reports, 13(5), 865-873.
https://doi.org/10.1016/j.celrep.2015.09.056
Harvard University, USA; Broad Institute of MIT and Harvard, USA and Hubrecht Institute, Netherlands.
2014:
Cultivation of microalgae (Chlorella vulgaris Beyerinck) in laboratory bioreactor LAMBDA MINIFOR
Heitur, H. (2014). Mikrovetika Chlorella vulgaris Beyerincki kasvatamine CO2 sidumise eesmärgil (Master's thesis).
https://hdl.handle.net/10492/1842 (2024 Feb. 05)
Eesti Maaülikool (Estonian University of Life Sciences), Estonia.
Growing yeast cultures (DBY12007) in the LAMBDA MINIFOR fermenter at steady state to study the aerobic glycolysis and energy flux
Slavov, N., Budnik, B. A., Schwab, D., Airoldi, E. M., & van Oudenaarden, A. (2014). Constant growth rate can be supported by decreasing energy flux and increasing aerobic glycolysis. Cell reports, 7(3), 705-714.
https://doi.org/10.1016/j.celrep.2014.03.057
Massachusetts Institute of Technology, USA; Harvard University, USA; Hubrecht Institute, Royal Netherlands Academy of Arts and Sciences and University Medical Center Utrecht, Netherlands and Princeton University, USA.
Selective and non-selective batch fermentation of date extract using Saccharomyces cerevisiae (commercial strain used in bakeries (wild strain), glucose selective strains ATCC 36858 and ATCC 36859) studied in LAMBDA MINIFOR fermentor
Putra, M. D., Abasaeed, A. E., Zeinelabdeen, M. A., Gaily, M. H., & Sulieman, A. K. (2014, April). Selective fermentation of pitted dates by S. cerevisiae for the production of concentrated fructose syrups and ethanol. In Journal of Physics: Conference Series (Vol. 495, No. 1, p. 012034). IOP Publishing.
https://doi.org/10.1088/1742-6596/495/1/012034
King Saud University, Chemical Engineering Department, Saudi Arabia
The metabolic stress response of tomato cell culture (Lycopersicum esculentum) to low oxygen studied using LAMBDA MINIFOR Bioreactor
Ampofo‐Asiama, J., Baiye, V. M. M., Hertog, M. L. A. T. M., Waelkens, E., Geeraerd, A. H., & Nicolai, B. M. (2014). The metabolic response of cultured tomato cells to low oxygen stress. Plant Biology, 16(3), 594-606.
https://doi.org/10.1111/plb.12094
KU Leuven, Belgium; Flanders Centre of Postharvest Technology (VCBT), Leuven, Belgium.
LAMBDA MINIFOR bioreactor to grow the oral bacteria (Streptococcus oralis, Actinomyces naeslundii, Veillonella parvula, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis) under planktonic conditions
Blanc, V., Isabal, S., Sanchez, M. C., Llama‐Palacios, A., Herrera, D., Sanz, M., & León, R. (2014). Characterization and application of a flow system for in vitro multispecies oral biofilm formation. Journal of Periodontal Research, 49(3), 323-332.
https://doi.org/10.1111/jre.12110
DENTAID S. L., Cerdanyola del Vallès, Spain; ETEP Research Group, University Complutense of Madrid, Spain.
LAMBDA MINIFOR Bioreactor used for recombinant protein (Chemokines) expression in E. coli
Kramp, B. (2014). Establishing the interaction between the CC chemokine ligand 5 and the receptors CCR1 and CCR (Doctoral dissertation, Aachen, Techn. Hochsch., Diss., 2013).
https://core.ac.uk/download/pdf/36589112.pdf (2024 Feb. 05)
RWTH Aachen, Germany.
Medium optimization and growth kinetics of S. marcescens were studied in a benchtop bioreactor LAMBDA MINIFOR 7L Advanced Kit: batch cultivations at 30 °C, 3 L/min aeration, 4 Hz stirring, pH 6, polypropylene glycol as antifoam.
Mohammed, S. J. (2014). Optimization Of medium Composition for increasing prodigiosin production by local isolate of Serratia marcescens. Doctoral thesis, Al-Khwarizmi College of engineering, Department of Biochemical engineering, University of Baghdad.
https://doi.org/10.13140/RG.2.2.18638.7968
2013:
Development of biofilm model (S. oralis, A. naeslundii, V. parvula, F. nucleatum, A. actinomycetemcomitans and P. gingivalis) in continuous flow in a LAMBDA MINIFOR 0.4L bioreactor.
Soto, I. S. (2013). Desarrollo del modelo de boca artificial en flujo continuo en el biorreactor Lambda Minifor. Universidad Complutense de Madrid Master en Ciencias Odontológicas.
https://docta.ucm.es/rest/api/core/bitstreams/39d8318b-a49b-4cb3-a164-e4faab18f52b/content (2024 Feb. 05)
Recombinant expression of the Met-CCL5, protease resistant CXCL12 (S4V) and F1-CX3CL1 in E. coli using LAMBDA MINIFOR fermenter/bioreactor to study their role in Cardiovascular disease (CVD)
Projahn, D. (2013). Generation, function and therapeutic application of chemotactic cytokines in cardiovascular diseases (Doctoral dissertation, Aachen, Techn. Hochsch., Diss., 2013).
https://publications.rwth-aachen.de/record/229207/files/4840.pdf (2024 Feb. 05)
RWTH Aachen, Germany.
Expression of Caf1 protein using Escherichia coli strain in LAMBDA MINIFOR fermentor to study mammalian cell adhesion, shape and number of focal adhesion
Machado Roque, A. I. (2013). Protein scaffolds for cell culture (Doctoral dissertation, Newcastle University).
URI: https://hdl.handle.net/10443/1843 (2024 Feb. 05)
Newcastle University, UK.
Controlled growth of Staphylococcus aureus under various concentrations of BAC (benzalkonium chloride) in LAMBDA MINIFOR fermentor
Cervinkova, D., Babak, V., Marosevic, D., Kubikova, I., & Jaglic, Z. (2013). The role of the qacA gene in mediating resistance to quaternary ammonium compounds. Microbial Drug Resistance, 19(3), 160-167.
https://doi.org/10.1089/mdr.2012.0154
Veterinary Research Institute, Brno, Czech Republic.
2012:
Effective production of Biobutanol from agricultural waste (giant hogweed, hay) using LAMBDA MINIFOR bench-top laboratory fermenter
Mezule, L., Tihomirova, K., Nescerecka, A., & Juhna, T. (2012). Biobutanol production from agricultural waste: A simple approach for pre-treatment and hydrolysis. Latvian Journal of Chemistry, 51(4), 407.
https://doi.org/10.2478/v10161-012-0028-5
2011:
Bioethanol production using Yeast (S. cerevisiae) in LAMBDA MINIFOR fermenter
Burešová, I., & Hřivna, L. (2011). Effect of wheat gluten proteins on bioethanol yield from grain. Applied Energy, 88(4), 1205-1210.
https://doi.org/10.1016/j.apenergy.2010.10.036
2010:
Anaerobic fermentation of the glucose component in dates extract by yeast Saccharomyces cerevisiae
Gaily, M. H., Elhassan, B. M., Abasaeed, A. E., & Al-Zahrani, S. M. (2010). A direct process for the production of high fructose syrups from dates extracts. International Journal of Food Engineering, 6(3).
https://doi.org/10.2202/1556-3758.1879
King Saud University, Saudi Arabia; University of Khartoum, Sudan
Study of the potential of tree tobacco stems (Nicotiana Glauca r. Grah.) as a bioethanol feedstock with the LAMBDA MINIFOR fermenter
Sánchez, F., Curt, M. D., Barreiro, M., Fernández, J., Agüera, J. M., Uceda, M., & Zaragoza, G. (2010). TREE TOBACCO (NICOTIANA GLAUCA R. GRAH.) STEMS AS A BIOETHANOL FEEDSTOCK. 18th European Biomass Conference and Exhibition, 3-7 May 2010, Lyon, France.
Do you sell/ship to the USA?
Yes, we do supply our instruments directly with door-to-door delivery option by the parcel services to the USA.
What is the availability of the product?
We have the instruments in stock. We would just have to configure the instruments according to your requirements and perform quality control before shipping.
Is there a warranty?
We offer a 2 year warranty for MINIFOR fermentor / bioreactor and 5 year warranty for the PRECIFLOW & MULTIFLOW peristaltic pumps.
Does this fermentor work on both mammalian cells and yeast cells?
Yes, MINIFOR fermentor and bioreactor can be used for mammalian and yeast cell cultures (More information at www.fermentor.net/applications).
Is there flexibility in the top plate to add or remove probes?
Yes, MINIFOR has free ports in the headspace for the additional probes (sensors). Multiple ports and other effective solutions in the fermentation glass vess make the MINIFOR configuration equivalent to 16 to 22 classical ports (it is possible to increase the number of ports – custom made solution)
Is the equipment suitable for use in pure / mixed culture?
Yes, MINIFOR is suitable for pure as well as mixed culture. The stirrer is strong and can easily be adapted according to the types of cultures and working volumes.
Why is MINIFOR perfectly suitable for parallel processes?
Each unit stays independent as it is equipped with a control panel and display and at a single glance shows the parameter values. All parameters are regulated locally inside each fermenter-bioreactor unit.
This allows fast and precise parameter regulation and never having to worry about leaving a vessel unattended. Further advantage is that in case there are problems with one unit, the other units will still keep running.
How important is the slowdown in parameter regulation while running 12 bioreactors in parallel?
An important aspect to consider – which, however, does not play a role in the LAMBDA MINIFOR parallel system because each MINIFOR fermenter comes with its proper regulation unit that measures and controls all parameters locally. As a consequence the quality of the measurement and regulation is not affected by long transmission times and dead times in regulation.
How much space is required for the MINIFOR unit?
Footprint: approximately a sheet of paper
Dimensions: 22 cm x 38 cm x 40 cm (W x H x D)
 Scale module for continuous cultures
Detaylı bilgi talebi
Scale module for continuous cultures
Detaylı bilgi talebi
 REDOX potential measurement [mV]
Detaylı bilgi talebi
REDOX potential measurement [mV]
Detaylı bilgi talebi
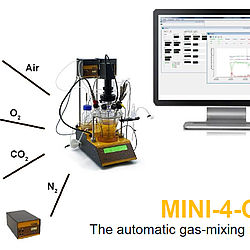 MINI-4-GAS Automatic gas-mix
Detaylı bilgi talebi
MINI-4-GAS Automatic gas-mix
Detaylı bilgi talebi
 Automatic antifoam control
Detaylı bilgi talebi
Automatic antifoam control
Detaylı bilgi talebi
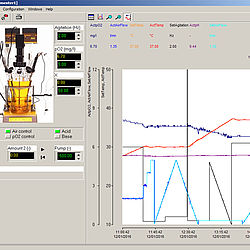 FNet - Fermentor Control Software
Detaylı bilgi talebi
FNet - Fermentor Control Software
Detaylı bilgi talebi
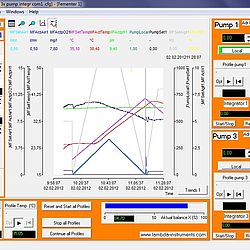 SIAM industrial fermentation software
Detaylı bilgi talebi
SIAM industrial fermentation software
Detaylı bilgi talebi
 MINI-4-GAS software module
Detaylı bilgi talebi
MINI-4-GAS software module
Detaylı bilgi talebi
 OXYMETER
O2 concentration measurement (0-25%)
Detaylı bilgi talebi
OXYMETER
O2 concentration measurement (0-25%)
Detaylı bilgi talebi
 CARBOMETER
CO2 concentration measurement (0-100%)
Detaylı bilgi talebi
CARBOMETER
CO2 concentration measurement (0-100%)
Detaylı bilgi talebi
 METHAMETER
CH4 concentration measurement (0-100%)
Detaylı bilgi talebi
METHAMETER
CH4 concentration measurement (0-100%)
Detaylı bilgi talebi
 Additional PRECIFLOW pump line
PRECIFLOW pump 0-600 ml/h, reagent bottle with pipes, fittings, filter, tubing
Detaylı bilgi talebi
Additional PRECIFLOW pump line
PRECIFLOW pump 0-600 ml/h, reagent bottle with pipes, fittings, filter, tubing
Detaylı bilgi talebi
 Additional MULTIFLOW pump line
MULTIFLOW pump, reagent bottle with pipes, fittings, filter and tubing
Detaylı bilgi talebi
Additional MULTIFLOW pump line
MULTIFLOW pump, reagent bottle with pipes, fittings, filter and tubing
Detaylı bilgi talebi
