Fermentador y biorreactor
Fermentador y biorreactor
Innovador fermentador / biorreactor de gran calidad a la mitad del costo. Nuevo concepto en fermentación y cultivo celular
Minifor2Bio - fermentador con pantalla táctil
Fermentador LAMBDA MINIFOR - Descripción de fermentador de laboratorio
Fermentador MINIFOR: Mediante la introducción de varias ideas e innovaciones hemos logrado producir un excelente fermentador-biorreactor a la mitad del costo, sin comprometer su calidad.

- Nuevo frasco o vaso de reacción de vidrio con cuellos laterales con rosca y un soporte de fijación
- Nuevo mezclador vibrador con membrana de silicona (vibromixing) en lugar de un costoso agitador magnético en hélice, que garantiza una esterilidad a largo plazo
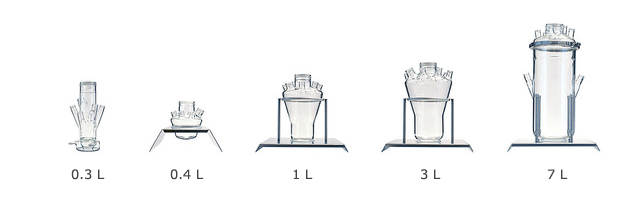
- Volúmenes de cultivo desde 35 mL hasta más 6 litros en un sólo equipo
- El nuevo radiador patentado IR (infrarrojo) con un reflector parabólico bañado en oro se utiliza para calentar suavemente el medio de cultivo (sin costosos baños termostáticos)
- Materiales de alto rendimiento reemplazan las costosas piezas de acero inoxidable en otros fermentadores
- Compacto y práctico, de fácil acceso desde cualquier ángulo
- Nueva agitación “fish-tail” (cola de pez) para suave mezclado en cultivos celulares
- Para cultivos continuos, procesos batch y fed-batch
- Indicado para la fermentación en paralelo
- Regulación precisa de gas por caudalímetro másico
- Control automático de formación de espuma (opcional)
- Montaje y desmontaje en tiempo mínimo
- Fácil sistema de esterilidad (“easy sterility”)
- Fácil operación y programación
- Esterilizable en autoclave común
- Autónomo o controlable por PC (FNet o SIAM)
La necesidad de un pequeno fermentador de laboratorio para volúmenes desde 0.035 hasta más de 6 litros nos llevó a la fabricación del MINIFOR.
Con base en nuestra vasta experiencia en fermentación, quisimos desarrollar un fermentador que fuera fácil de usar y que tuviera la capacidad de medir y controlar todos los parámetros importantes requeridos en un cultivo biológico. Para cumplir dicha función, el fermentador debe ocupar un mínimo de espacio en el laboratorio, pero dejando buen acceso a todas sus partes.
Para la optimización de los parámetros de crecimiento del cultivo o de la biotransformación, debe ser posible colocar varios fermentadores uno al lado del otro. Cada fermentador debe ser capaz de trabajar independientemente o conectado a un PC, para una avanzada regulación y procesamiento de datos.
Con el fin de mantener el bajo costo del Fermentador MINIFOR, hemos introducido varias ideas e innovaciones sin comprometer su calidad:
En lugar de un frasco con una costosa cubierta de acero inoxidable, utilizamos un recipiente o vaso de reacción de vidrio con cuellos laterales con rosca, sistema que ha sido utilizado por muchos anos en el área del cultivo celular para mantener la esterilidad.
En lugar del tradicional agitador de hélice, que requiere de un costoso motor y acoplamiento magnético, hemos introducido un nuevo sistema de vibración. Un electroimán y una económica membrana aseguran una perfecta esterilidad y garantizan una mezcla efectiva, sin la formación de vórtices en el medio. No se precisan baffles (deflectores). Al mismo, tiempo este tipo de mezclador es más suave con las células y produce menos espuma.
El cultivo es calentado a través de la radiación de calor producida por un radiador con un reflector parabólico bañado en oro, localizado debajo del recipiente fermentador. De esta manera el cultivo es calentado suavemente de modo similar al que el sol calienta el agua. Así no habrá sobrecalentamiento del cultivo, como generalmente ocurre cuando el calentador está colocado dentro del medio. Los costosos frascos de doble pared y baños termostáticos quedan eliminados, al mismo tiempo que los tubos y cables, haciendo al fermentador menos complejo.
Con la implementación de modernos microprocesadores, ha sido posible situar toda su electrónica en la parte frontal del aparato, eliminando así la carcasa de los fermentadores tradicionales. Esto hace al fermentador increíblemente compacto. A pesar de su pequeno tamano, el MINIFOR tiene la capacidad de medir y controlar 6 parámetros en la configuración básica del MINIFOR.
Dimensiones: 22 x 40 x 38 cm (A x D x H)
Visualizador: LCD de 4 x 40 dígitos con iluminación de fondo
Frasco fermentador: Vasos de 0,3, 0,4, 1,3, y 6 L de vidrio de borosilicato Pyrex con 6-8 cuellos con roscas
Control de temperatura: Fuente de calor mediante radiaciones infrarrojas (IR) de alta eficiencia de 150 W con reflector parabólico dorado
Regulación: Desde 5°C sobre la temperatura ambiente hasta 70°C
Medición: Desde 0 hasta 99.9°C en pasos de 0.1°C
Precisión: +/- 0.2°C (0 hasta 60°C)
Sensor: Pt 100 incorporada en el electrodo de cristal o vidrio de electrodo de pH
Control del pH: Un electrodo de pH 0 - 14 esterilizable, con corrección automática de la temperatura, calibración semiautomática de dos puntos y conector Variopin
Resolución: 0.01 pH unidades
Precisión: +/- 0.02 pH unidades
Control pO2: El electrodo de oxígeno tipo Clark esterilizable con una respuesta rápida, corrección automática de la temperatura, calibración semiautomática de dos puntos, y control de oxígeno disuelto (OD) a través de la regulación del flujo de aire
Rango: 0 hasta 25 mg de oxígeno/l, en pasos de 0.1 mg/l
Flujo de aire: De 0 a 5 l/min en pasos de 0,01 l/min, medido por un preciso de flujo de masa, linealidad +/- 3%, reproducibilidad +/- 0,5%
Control: Válvula proporcional controlada por microprocesador
Presión del aire: 0.05 – 0.2 MPa (0.5 - 2 atm)
Agitación: Vibromezclador de 50 W de 0 a 20 Hz (de 0 a 1200 rpm) en intervalos o pasos de 0,1 Hz (6 rpm) con 1 o más discos de agitación; esterilidad similar al acoplamiento magnético
Parámetro adicional: Un parámetro adicional puede ser controlado por el instrumento (formación de espuma, el peso (para cultivos continuos), pCO2, potencial redox, conductividad, etc); con normas o estándares de salida de 0-10V o 0-20mA
Ports / side necks: Un puerto de muestreo cuádruple o para adiciones con 4 agujas con conexiones de LAMBDA-PEEK con doble sello usados para muestreo, inoculación, anti-espumante, suministro de medio, etc, puertos adicionales dobles están disponibles
Bombas: Hasta 4 bombas independientes (PRECIFLOW, MULTIFLOW, HIFLOW o MAXIFLOW) con variación de la velocidad de 0 a 100% pueden ser utilizadas con el bioreactor - fermentador de laboratorio MINIFOR
Control de flujo de gas: Además de las bombas, varios controladores electrónicos de flujo con intervalos de flujo de 0 - 5 l/min (MASSFLOW 5000) ó 0 - 500 ml/min (MASSFLOW 500) puede usarse para la adición regulada de los gases (Ej, N2, O2, aire, CO2) en cultivos celulares; módulo de estación de gases de configuración libre
Temperatura de trabajo: 0 - 40 °C
Humedad relativa: 0 - 90 % RH, sin condensación
Peso: 7.5 kg
Control PC: Control completo mediante el ordenador y el procesamiento de datos de la fermentación utilizando el programa FNet (para un máximo de 6 fermentadores MINIFOR) o SIAM (para un número aún mayor de instrumentos)
- EN - LAMBDA MINIFOR Laboratory Fermenter-Bioreactor LEAFLET (pdf)
- EN - 20 innovations of LAMBDA MINIFOR laboratory-scale bioreactors — Overview and Benefits (pdf)
- EN - LAMBDA MINIFOR laboratory fermenter and bench-top bioreactor at universities and highschools (pdf)
- EN - Operation manual: MINIFOR lab fermentor-bioreactor (pdf)
- EN - Infographics: Overview of innovations in MINIFOR lab fermenter and bioreactor (pdf)
- EN - Quick overview of MINIFOR bioreactor vessels with connections, probes & tubing lines (pdf)
- EN - Peltier electronic cooling loop for Minifor fermentor (pdf)
- EN - PUMP-FLOW INTEGRATOR replaces the need for optical density measurements in fermenters and bioreactors (pdf)
- EN - REDOX potential measurement for MINIFOR laboratory fermentor (pdf)
- EN - MINI-4-GAS, the automatic gas mixing module (pdf)
- EN - How to compare the cost and real value of a laboratory fermentor? (pdf)
- EN - Solitary teamplayer in parallel processes - LAMBDA MINIFOR laboratory fermenter and bioreactor (pdf)
- DE - UNI _ LAMBDA MINIFOR Laborfermenter und Tischbioreaktor an Hochschulen (pdf)
- DE - Montageanleitung _ Kurzübersicht der Bioreaktorgefässe LAMBDA MINIFOR mit Anschlüssen, Sonden und Schlauchlinien (pdf)
- DE - Broschüre _ MINIFOR Laborfermenter Bioreaktor (pdf)
- DE - Parallelreaktor _ LAMBDA MINIFOR Laborfermenter Bioreaktor der Einzelgänger im Parallelsystem (pdf)
- DE - Bedienungsanleitung _ LAMBDA MINIFOR Labor-Tischfermenter und Bioreaktor (pdf)
- DE - LAMBDA MINIFOR Laborfermenter und Bioreaktor - Innovationsuebersicht und Vorteile (pdf)
- FR - MINIFOR - fermenteur de laboratoire (pdf)
- FR - Installation de cuve LAMBDA MINIFOR (pdf)
- FR - LAMBDA MINIFOR Start-Up Kit - mode d'emploie (pdf)
- FR - LAMBDA MINIFOR fermenteur / bioreacteur - Resume des innovations et avantages (pdf)
- FR - LAMBDA MINIFOR fermenteur de laboratoire-bioréacteur de paillasse et son utilisation dans les écoles et universités (pdf)
- FR - Le fermenteur et bioréacteur de laboratoire MINIFOR: Le joueur solitaire dans l’équipe des procédés en parallèle (pdf)
- FR - Comment comparer le coût d’un fermenteur ou bioréacteur avec sa valeur réelle ? (pdf)
- RU - Лабораторный ферменторбиореактор ЛАМБДА МИНИФОР (pdf)
- CZ - MINIFOR - laboratorní fermentor (pdf)
- ES - Manual de operaciones - LAMBDA MINIFOR Fermentador-Biorreactor de Laboratorio (pdf)
- ES - LAMBDA MINIFOR Biorreactor-fermentador de laboratorio - resumen de innovaciones y ventajas (pdf)
- ES - Fermentador-biorreactor de laboratorio LAMBDA MINIFOR para las escuelas y universidades (pdf)
- ES - MINIFOR - fermentador de laboratorio (pdf)
- ES - Fermentador y biorreactor LAMBDA MINIFOR: El solitario en procesos paralelos (pdf)
- ES - ¿Cómo comparar el coste y el valor real de un fermentador y biorreactor de laboratorio? (pdf)
2024
Sacarificación y fermentación simultánea (SSF): El biorreactor LAMBDA MINIFOR se utilizó tanto para la fermentación de biomasa sólida pretratada (10 %) en presencia de celulasa, β-glucosidasa e inoculada con Lactobacillus rhamnosus (1.7 L, 37 °C, pH=5.5, 72 h), como para la posterior fermentación de hidrolizados con Bacillus megaterium (1.7 L, 35 °C, 48 h).
Senila, L., Kovacs, E., Resz, M. A., Senila, M., Becze, A., & Roman, C. (2024). Life Cycle Assessment (LCA) of Bioplastics Production from Lignocellulosic Waste (Study Case: PLA and PHB). Polymers 2024, 16, 3330.
https://doi.org/10.3390/polym16233330
Cultivo anaeróbico por lotes de C. butyricum en un fermentador LAMBDA MINIFOR 3L, purgado con argón antes de la fermentación: pH 5.6 (controlado con NaOH 1M), 37 °C y 6000 rpm. La producción de gas se midió por el método de desplazamiento de agua, el volumen de agua desplazada se controló con una cámara. Se recogieron muestras de líquido y gas a intervalos..
Pištěková, H., Dusankova, M., Sopik, T., Klaban, J., Dostalkova, J., Moučka, R., & Sedlarik, V. Optimization of the Dark Fermentation Technique for Hydrogen Production Through Supplementation with Ascorbic Acid And/Or L-Cysteine by Clostridium Butyricum Ccdbc 11. Or L-Cysteine by Clostridium Butyricum Ccdbc, 11.
https://dx.doi.org/10.2139/ssrn.4973469
Fermentaciones alcohólicas ensayadas en condiciones de microvinificación en un biorreactor LAMBDA MINIFOR con parámetros controlados (T = 20 °C, frecuencia de agitación = 2 Hz) y condiciones estándar (T = 12 - 14 °C).
Filimon, G. (2024). Monitorizarea fermentaţiei alcoolice a musturilor obţinute din soiuri de struguri rizogene. Universitatea Tehnică a Moldovei. Technical Scientific Conference of Undergraduate, Master and PhD Students: Chişinău, 27-29 martie 2024. Vol. 2.
URI: http://repository.utm.md/handle/5014/28059
Biodegradación: Tratamiento de muestras marinas con adición de «Diesel-B5» (10 %, 15 %, 20 %) como fuente de C en biorreactores aireados y agitados LAMBDA MINIFOR (Pseudomonas sp.; pH 6,5; temperaturas de incubación 25 °C, 35 °C y 45 °C).
Peña Ventura, J. C. (2024). Determinación del ratio de biodegradación de hidrocarburos totales de petróleo empleando Pseudomonas sp. en agua de mar contaminada con diésel-B5. Universidad Nacional de Trujillo, Unidad de Postgrado en Ingeniería Química, Tesis de maestro en ingeniería química ambiental.
URI: https://hdl.handle.net/20.500.14414/22166
El inóculo mezclado se inoculó en la cámara de reacción del biorreactor LAMBDA MINIFOR, que se mantuvo a 37 °C en condiciones anaerobias.
Khan, S. N., Ribeiro‐Vidal, H., Virto, L., Bravo, E., Nuevo, P., Koldsland, O. C., Hjortsjö, C. & Sanz, M. (2024). The Decontamination Effect of an Oscillating Chitosan Brush Compared With an Ultrasonic PEEK‐Tip: An In Vitro Study Using a Dynamic Biofilm Model. Clinical Oral Implants Research.
https://doi.org/10.1111/clr.14360
Desarrollo in vitro de un modelo dinámico de biopelícula multiespecie en superficies de implantes: Crecimiento planctónico de una mezcla de seis cepas bacterianas diferentes en el interior del biorreactor LAMBDA MINIFOR (12 h), seguido de cultivo continuo (flujo de 30 mL/h) a través de 2 dispositivos Robbins que contienen los implantes estériles (37 °C, anaerobiosis, 72 h).
Khan, S. (2024). Efficacy of Mechanical Decontamination Strategies in the Treatment of Peri-implantitis. Doctoral thesis, University of Oslo.
URI: https://hdl.handle.net/10852/111365 (2024 August 07)
La fermentación en co-cultivo por lotes de jarabe de palmera datilera con S. cerevisiae y P. stipitis ATCC 58785 se llevó a cabo con el kit avanzado del fermentador de tanque agitado LAMBDA MINIFOR 1L.
Shalsh, F. J., Gatte, E. H., Alsultan, I. I., Tariq, F. Z., Jassim, S. M., Saleh, M. T., & Alrazzaq, N. A. (2024). Bioplastic Compounds of Succinic Acid from Agriculture Waste; Date Palm Syrup And Date Palm Fronds. Nepal Journal of Biotechnology, 12(1), 32-39.
https://doi.org/10.54796/njb.v12i1.310
Modelo dinámico in vitro de biopelícula multiespecie: Durante todo el proceso de incubación (crecimiento de biopelículas sobre superficies de implantes), el biorreactor LAMBDA MINIFOR mantiene el medio de cultivo a 37 °C, pH 7.2 y una atmósfera anaeróbica (10% H2, 10% CO2 y balance N2).
Bravo, E., Arce, M., Ribeiro-Vidal, H., Herrera, D., & Sanz, M. (2024). The Impact of Candida albicans in the Development, Kinetics, Structure, and Cell Viability of Biofilms on Implant Surfaces—An In Vitro Study with a Validated Multispecies Biofilm Model. International Journal of Molecular Sciences, 25(6), 3277.
https://doi.org/10.3390/ijms25063277
Fermentaciones por lotes sumergidos (consorcio) y por lotes alimentados (L. coryniformis) en biorreactor de tanque agitado LAMBDA MINIFOR y software SIAM: 72 h, volúmenes de trabajo finales = 200 ml, T = 37 ± 0.5 °C, agitación 0.5 Hz, CO2 a 0.04 L/min, pH 6.5 ± 0.5 utilizando NaOH 1 M y H2SO4 1 M para el ajuste automático del pH; se tomaron muestras a intervalos regulares.
Buljubašić, E., Bambace, M. F., Christensen, M. H. L., Ng, K. S., Huertas‐Díaz, L., Sundekilde, U., Marietou, A. & Schwab, C. (2024). Novel Lactobacillaceae strains and consortia to produce propionate‐containing fermentates as biopreservatives. Microbial Biotechnology, 17(2), e14392.
https://doi.org/10.1111/1751-7915.14392
Se utilizó el modelo de biopelícula dinámica multiespecie in vitro, que ha sido validado en biopelículas que crecen en superficies de implantes: El biorreactor LAMBDA MINIFOR mantiene el medio BHI enriquecido con proteínas en condiciones estables: 37 °C, pH 7.2 y una atmósfera anaeróbica mediante el bombeo directo de una mezcla de gases anaeróbicos (10% H2, 10% CO2 y N2 en equilibrio) manteniendo la presión constante durante todo el proceso de incubación.
Bravo, E., Arce, M., Ribeiro-Vidal, H., Herrera, D., & Sanz, M. (2024). The Impact of Candida albicans in the Development, Kinetics, Structure, and Cell Viability of Biofilms on Implant Surfaces. An In Vitro Study with a Validated Multispecies Biofilm Model.
https://doi.org/10.20944/preprints202402.1137.v1
Modelo simulación de colon humano con el LAMBDA MINIFOR usando medio nutritivo basal (37 °C, pH 6.8) y lodos fecales
Pusuntisumpun, N., Tunsagool, P., Nitisinprasert, S., & Nakphaichit, M. (2024). Impacts of combining Limosilactobacillus reuteri KUB‐AC5 and Limosilactobacillus fermentum KUB‐D18 on overweight gut microbiota using a simulated human colon model. International Journal of Food Science & Technology.
https://doi.org/10.1111/ijfs.16941
Se inyectaron 400 ml de células aisladas de tomate en un biorreactor LAMBDA MINIFOR (20 °C, pH 5.8) (10 L/h) para alcanzar distintas concentraciones de O2 (21 kPa, 5 kPa y 0 kPa) en el estudio de las pérdidas poscosecha de frutas y hortalizas durante el almacenamiento en atmósfera controlada como resultado del estrés por bajo nivel de O2.
Mahomud, M. S., Islam, M., & Roy, J. (2024). Effect of low oxygen stress on the metabolic responses of tomato fruit cells. Heliyon, e24566.
https://doi.org/10.1016/j.heliyon.2024.e24566
2023
Hülberné Beyer, É. A., Nemestóthy, N., & Bélafiné Bakó, K. (2023). Case Study of Continuous Itaconic Acid Fermentation by Aspergillus Terreus in a Bench-Scale Bioreactor. Hungarian Journal of Industry and Chemistry, 51(2), 57-63.
Sacarificación simultánea (hidrólisis enzimática con celulasa y β-glucosidasa) y fermentación (Lacticaseibacillus rhamnosus) de biomasa lignocelulósica de ciruela delignificada pretratada a ácido láctico en el biorreactor LAMBDA MINIFOR Advanced Kit (1.7 L; 37 °C /44 °C; pH 5.5 / 6.5; 72 h).
Senila, L., Cadar, O., Kovacs, E., Gal, E., Dan, M., Stupar, Z., Simedru, D., Senila, M. & Roman, C. (2023). L-Poly(lactic acid) Production by Microwave Irradiation of Lactic Acid Obtained from Lignocellulosic Wastes. Int. J. Mol. Sci. 2023, 24, 9817.
Senila, L., Gál, E., Kovacs, E., Cadar, O., Dan, M., Senila, M. & Roman, C. (2023). Poly(3-hydroxybutyrate) Production from Lignocellulosic Wastes Using Bacillus megaterium ATCC 14581. Polymers. 2023, 15, 4488.
Bravo, E., Serrano, B., Ribeiro-Vidal, H., Virto, L., Sánchez, I.S., Herrera, D. & Sanz, M. (2023). Biofilm formation on dental implants with a hybrid surface microtopography: An in vitro study in a validated multispecies dynamic biofilm model. John Wiley & Sons, Ltd., 0905-7161. Clinical Oral Implants Research, Volume 34, Issue 5, May 2023 Pages i-iii, 405-541.
Simulación de un modelo intestinal in vitro con LAMBDA MINIFOR 0,3L: fermentaciones por lotes en condiciones anaeróbicas (37 °C, pH 6.8–6.9; 24 h) de suspensión fecal humana (1 % (v/v)) para evaluar el efecto de Triphala Extractos (1 mg/ml) cofermentación sobre microbiota y cambios metabólicos.
Kwandee, P., Somnuk, S., Wanikorn, B., Nakphaichit, M. & Tunsagool, P. (2023). Efficacy of Triphala extracts on the changes of obese fecal microbiome and metabolome in the human gut model. Journal of Traditional and Complementary Medicine, Volume 13, Issue 2, 2023, Pages 207-217, ISSN 2225-4110.
Modelo de Simulación Gastrointestinal SHIME con reactores LAMBDA MINIFOR 0.3L:
Cada simulador del ecosistema microbiano intestinal humano consta de cinco reactores, que imitan diferentes secciones del tracto gastrointestinal humano.
El video https://www.youtube.com/watch?v=hXcpa0bXu6Q muestra a Assoc. Prof. Massalin Nakphaichit frente a los reactores SHIME. Departamento de Biotecnología, Facultad de Agroindustria, Universidad Kasetsart.
En un biorreactor de laboratorio LAMBDA MINIFOR 7L con sondas programables, se desarrollaron RODMs (un conjunto de organismos con un metabolismo novedoso que degradan eficientemente aromáticos altamente concentrados) para un ecosistema microbiano de alta densidad.
Ahmad, M., Yousaf, M., Han, J.-C., Huang, Y., Zhou, Y. & Tang, Z. (2023). Development of Biocatalytic Microbial Ecosystem (FPUS@RODMs@In-PAOREs) for Rapid and Sustainable Degradation of Various Refractory Organics. Journal of Hazardous Materials, 2023, 131514, ISSN 0304-3894,
https://doi.org/10.1016/j.jhazmat.2023.131514
Célula de hibridoma (inoculación 4.0×10E5 células/mL (90% de viabilidad)): Alimentación por lotes en un biorreactor de tanque agitado LAMBDA MINIFOR.
Llamo, A., Hernández, D., García, C., González, M., Ferro, W., Garay, H., Diago, D., Fajardo, A., Espinosa, L., Padilla, S., Gómez, L., Chinea, G. & and Valdés, R. (2023). Gamma-Immunoglobulin Response Characterization, in COVID-19 Convalescent Patients, Against the Spike Protein S2 Subunit with Eight Linear Peptides for Monoclonal Antibody Generation. BioProcess J, 2023; 22.
https://doi.org/10.12665/J22OA.Llamo
Optimización de la hidrólisis de aceites vegetales catalizada por lipasas: Control y seguimiento mediante el biorreactor LAMBDA MINIFOR (pH, temperatura, tiempo de reacción, carga enzimática y relación aceite/acuosa de la mezcla de reacción).Faillace, E., Brunini-Bronzini de Caraffa, V., Mariani, M., Berti, L., Maury, J. & Vincenti, S. (2023). Optimizing the First Step of the Biocatalytic Process for Green Leaf Volatiles Production: Lipase-Catalyzed Hydrolysis of Three Vegetable Oils. International Journal of Molecular Sciences. 2023; 24(15):12274.
Dumitru, M. & Ciurescu, G. (2023). Optimization of the fermentation conditions and survival of Bacillus licheniformis as freeze-dried powder for animal probiotic applications. Scientific Papers. Series D. Animal Science. Vol. LXVI, No. 2, 2023; ISSN 2285-5750; ISSN CD-ROM 2285-5769; ISSN Online 2393-2260; ISSN-L 2285-5750.
Alonso-Español, A., Bravo, E., Ribeiro-Vidal, H., Virto, L., Herrera, D., Alonso, B. & Sanz, M. (2023). The Antimicrobial Activity of Curcumin and Xanthohumol on Bacterial Biofilms Developed over Dental Implant Surfaces. Int. J. Mol. Sci. 2023, 24, 2335.
La leche se pasteurizó a 70 °C durante 30 minutos en fermentadores LAMBDA MINIFOR
/
Fermentadores LAMBDA MINIFOR: Pruebas de efecto de la temperatura en el crecimiento de la levadura Kazachstania unispora (inicial ~10E6 CFU/ml) en leche (800 ml, ~6 % lactosa): de 5 °C a 40 °C (5, 10, 15, 20, 25, 27, 30, 32, 35, 37, y 40 °C) a pH 5.6 (ajuste automático con 2 M NaOH) y 240 rpm, hasta que se alcanzó la fase estacionaria (sensor de turbidez en línea de infrarrojo cercano Optek FC20- ASD10-N).
/
Fermentadores LAMBDA MINIFOR para experimentos de cocultivo a 25 °C de Lacticaseibacillus casei y Kazachstania unispora en medios MRS modificados, así como en leche de yegua (inicial: ~10E6 UFC/ml, pH = 6.8)
/
Fermentadores LAMBDA MINIFOR para experimentos de cocultivo a 30 °C de Lactobacillus kefiri y Kazachstania unispora en medios MRS modificados, así como en leche de yegua (inicial: ~10E6 UFC/ml, pH = 6.8).
Kondybayev, A., Achir, N., Mestres, C., Collombel, I., Strub, C., Grabulos, J., Akhmetsadykov, N., Aubakirova, A., Kamidinkyzy, U., Ghanmi, W. & Konuspayeva, G. (2023). Growth Kinetics of Kazachstania unispora and Its Interaction with Lactic Acid Bacteria during Qymyz Production. Fermentation 2023, 9, 101.
https://doi.org/10.3390/fermentation9020101
Pasotti, L., De Marchi, D., Casanova, M., Frusteri Chiacchiera, A., Cusella De Angelis, M. G., Calvio, C., & Magni, P. (2023). Design of a stable ethanologenic bacterial strain without heterologous plasmids and antibiotic resistance genes for efficient ethanol production from concentrated dairy waste. Biotechnology for Biofuels and Bioproducts, 16(1), 1-13.
https://doi.org/10.1186/s12896-017-0369-y
2022:
www.geniusjournals.org/index.php/emrp/article/view/2162 (2022 Sept. 22)
Biorreactor y fermentador LAMBDA MINIFOR para escuelas y universidades:
Technologische Fachoberschule Bruneck, Fachrichtung Chemie, Werkstoffe und Biotechnologie (2021). Bioreaktor.
Experimentos de crecimiento de bacterias del ácido láctico: efecto de la temperatura en Lacticaseibacillus casei y Lactobacillus kefiri.
Kondybayev, A.; Konuspayeva, G.; Strub, C.; Loiseau, G.; Mestres, C.; Grabulos, J.; Manzano, M.; Akhmetsadykova, S. & Achir, N. (2022). Growth and Metabolism of Lacticaseibacillus casei and Lactobacillus kefiri Isolated from Qymyz, a Traditional Fermented Central Asian Beverage. Fermentation 2022, 8, 367.
Durante 65 días, se operaron continuamente dos fermentadores de tanque agitado(HRT= 5 días) LAMBDA MINIFOR en condiciones anaeróbicas (N2 en el espacio de cabeza y rociado), cada uno con un volumen de trabajo de 1 litro (modificación de las concentraciones de lactato / acetato) inoculados con lodo productor de caproato (género Caproiciproducens (familia Ruminococcaceae)), control de temperatura (30 °C, calentador IR incorporado, sonda Mettler InPro 3253) y control de pH (pH 5.5, NaOH 2M, HCl 0.5M)) con cuatro bombas peristálticas (alimentación, efluente , base y ácido) y muestreo líquido diario para análisis de carboxilatos y alcoholes.
Cultivo continuo: 1 mes en quimiostato con el fotobiorreactor LAMBDA MINIFOR PBR (1L; 30 °C; 1 L/min de aire enriquecido con CO2 (0.5%); 5 Hz, pH 8; luz blanca) con Synechocystis sp. PCC 6803
Behle, A., Dietsch, M., Goldschmidt, L., Murugathas, W., Berwanger, L.C., Burmester, J., Yao, L., Brandt, D., Busche, T., Kalinowski, J., Hudson, E.P., Ebenhöh, O., Axmann, I.M. & Machné, R. (2022). Manipulation of topoisomerase expression inhibits cell division but not growth and reveals a distinctive promoter structure in Synechocystis. Nucleic Acids Research, Volume 50, Issue 22, 9 December 2022, Pages 12790–12808.
https://doi.org/10.1093/nar/gkac1132
Resolución biocatalítica de racemato de lupanina en aguas residuales industriales por Pseudomonas putida LPK411 usando un biorreactor a escala de laboratorio LAMBDA MINIFOR0.4L en modo de operación discontinuo.
Parmaki, S., Esteves, T., Gonçalves, J.M.J. Catenacci, A., Malpei, F., Ferreira, F.C., Afonso C.A.M & Koutinas, M. (2022). Selective microbial resolution of lupanine racemate: Bioprocess development and the impact of carbon catabolite repression on industrial wastewater valorisation. Biomass Conv. Bioref. (2022).
https://doi.org/10.1007/s13399-022-03383-3
Para la liberación de péptidos bioactivos, proteina aislada de Kiwicha a partir de harina de semillas de Amaranthus caudatus L. fue sometida a hidrólisis enzimática con Bioproteasa LA-660 utilizando un biorreactor LAMBDA MINIFOR con presión continua, control de pH y temperatura (pH 8; 50 °C).
Martinez-Lopez, A., Rivero-Pino, F., Villanueva, A., Toscano, R., Grao-Cruces, E., Marquez-Paradas, E., Martin, M.E., Montserrat-de la Paz, S. & Millan-Linaresa, M.C. (2022). Kiwicha (Amaranthus caudatus L.) protein hydrolysates reduce intestinal inflammation by modulating the NLRP3 inflammasome pathway. Food & Function 2022 Oct 21.
https://doi.org/10.1039/D2FO02177C
Fermentador LAMBDA MINIFOR 7L en la producción de un alto volumen de vesículas de membrana externa (OMV) a partir de cepa mutante de Escherichia coli (E. coli; E44Δ).
Allahghadry, T., Bojesen, A.M., Whitehead, B.J. and Antenucci, F. (2022). Clarification of large-volume bacterial cultures using a centrifuge-free protocol. J Appl Microbiol. Accepted Author Manuscript.
https://doi.org/10.1111/jam.15608
2021:
Experimentos en fase líquida (hidrolizado de hemicelulosas) para la producción de xilitol: Se realizó la fermentación de 250 ml de hidrolizado detoxificado en un fermentador de 1L (LAMBDA MINIFOR de sobremesa-laboratorio-fermentador) y ajuste de pH (pH 5.0) en condiciones aeróbicas a 30° C durante 60 h.
Shalsh, D., Nagimm, D., Alrheem, M.A. & Alrheem, S.A. (2021). Batch fermentation and Simultaneous Saccharification and Fermentation (SSF) processes by Meyerozyma Guilliermondii Strain F22 and Saccharomyces cerecvisae for xylitol and bioethanol co-production. Al-Qadisiyah Journal of Pure Science, 26(4), 80–94.
https://doi.org/10.29350/qjps.2021.26.4.1347
Se modelaron el crecimiento, consumo de glucosa y producción de etanol de la cepa LM de Saccharomyces cerevisiae en caldo sintético para el intrínseco más importante. Se utilizaron fermentadores LAMBDA MINIFOR de un litro equipados con un condensador de agua fría en tuberías de salida de aire (LAMBDA Instruments GmbH, Baar- Suiza).
Kouamé, C., Loiseau, G., Grabulos, J., Boulanger, R. & Mestres, C. (2021). Development of a model for the alcoholic fermentation of cocoa beans by a Saccharomyces cerevisiae strain. International Journal of Food Microbiology, Volume 337, 2021, 108917, ISSN 0168-1605.
https://doi.org/10.1016/j.ijfoodmicro.2020.108917
Cultivo continuo de cianobacterias Synechocystis sp. PCC 6803 en un fotobiorreactor LAMBDA MINIFOR 1L
Behle, A., Dietsch, M., Goldschmidt, L., Murugathas, W., Brandt, D., Busche, T., Kalinowski, J., Ebenhöh, O., Axmann, I. M. & Machné, R. (2021) Uncoupling of the Diurnal Growth Program by Artificial Genome Relaxation in Synechocystis sp. PCC 6803. bioRxiv 2021.07.26.453758.
https://doi.org/10.1101/2021.07.26.453758
La hidrólisis del aislado de proteína de kiwicha (KPI) se realiza bajo agitación continua, utilizando un fermentador-biorreactor LAMBDA MINIFOR, en condiciones controladas de pH y temperatura: Se adiciona Bioproteasa LA-660 a razón enzima/sustrato = 0.3 AU/g proteína ( pH 8) durante 5, 10, 15, 30 y 60 min.
Paz, S. M. D. L., Martinez-Lopez, A., Villanueva-Lazo, A., Pedroche, J., Millan, F., & Millan-Linares, M. C. (2021). Identification and characterization of novel antioxidant protein hydrolysates from kiwicha (Amaranthus caudatus L.). Antioxidants, 10(5), 645.
https://doi.org/10.3390/antiox10050645
La transformación biológica de la biomasa de sorgo blanco se realizó en condiciones de operación similares al proceso MixAlco en el biorreactor LAMBDA MINIFOR.
Shalsh, F.J., Alrazzaq, N.A., Nagimm D.K., Alrheem, M.A., Alrheem S.A. & Abd-alalah, K. (2021). Bioconversion of white sorghum biomass using MixAlco fermentation process. DYSONA – Applied Science. 2021(2), 21-27. ISSN 2708-6283.
https://doi.org/10.30493/DAS.2021.248966
2020:
Se cultivaron diferentes cepas de levadura en el fermentador LAMBDA MINIFOR 0.4 L para estudiar el ciclo metabólico y la ruta: A) lote de 200 ml: medio YMC-YE / YMC-MD, pH 3.5, aireación de 0.15 L/min, 30 °C; B) inanición 6 h; C) cultivo continuo: velocidad de dilución de 0,082 1/h
J. Feltham, S. Xi, S. Murray, M. Wouters, J. Urdiain-Arraiza, C. George, A. Townley, E. Roberts, R. Fisher, S. Liberatori, S. Mohammed, B. Kessler & J. Mellor. (2020). Transcriptional changes are regulated by metabolic pathway dynamics but decoupled from protein levels. bioRxiv 833921.
https://doi.org/10.1101/833921
El biorreactor LAMBDA MINIFOR (equipado con 2 bombas peristálticas (bomba de saliva y permeado), un mezclador superior, una sonda de pH-temperatura redox, una unidad de control de temperatura y un módulo de membrana de fibra hueca sumergida) se utiliza como un biorreactor de membrana ruminal para producir compuestos volátiles. ácidos grasos (AGV) a partir de residuos de cultivos (biomasa lignocelulósica) imitando el sistema digestivo de los animales rumiantes.
Nguyen, A.Q., Nguyen, L.N., Abu Hasan Johir, M., Ngo, H-H., Chaves, A.V. & Nghiem, L.D. (2020) Derivation of volatile fatty acid from crop residues digestion using a rumen membrane bioreactor: a feasibility study. Bioresource Technology 2020.
https://doi.org/10.1016/j.biortech.2020.123571
Experimentos de hidrólisis enzimática se llevaron a cabo en el biorreactor de laboratorio LAMBDA MINIFOR. Los residuos de sarmientos pretratados se deslignificaron con clorito de sodio para eliminar la lignina y luego se hidrolizaron enzimáticamente utilizando nuevos tipos de enzimas (celulasa de Trichoderma reesei y b-glucosidasa).
Eniko Kovacs, Daniela Alexandra Scurtu, Lacrimioara Senila, Oana Cadar, Diana Elena Dumitras & Cecilia Roman (2020). Green Protocols for the Isolation of Carbohydrates from Vineyard Vine-Shoot Waste. Analytical Letters.
https://doi.org/10.1080/00032719.2020.1721001
Biorreactor LAMBDA MINIFOR utilizado para producir biotecnológicamente ácido itacónico a partir de glucosa por la cepa fúngica Aspergillus terreus: 1.8 L de medio con 120 g/L de glucosa como sustrato, modo discontinuo, aerobio: aireación 2 L/min (STP) = 6.5 mg/L inicial DO, 37 °C, agitación 2 Hz, pH 3)
Nemestóthy, N., Komáromy, P., Bakonyi, P. et al. (2020). Carbohydrate to Itaconic Acid Conversion by Aspergillus terreus and the Evaluation of Process Monitoring Based on the Measurement of CO2 Waste and Biomass. Valorization 2020.
https://doi.org/10.1007/s12649-019-00729-3
2019:
Biorreactor LAMBDA MINIFOR 0.4L con bombas de medio PRECIFLOW es utilizado en experimentos turbidostatos con células recombinantes en modo de operación de cultivo continuo.
Pasotti, L., Bellato, M., Politi, N., Casanova, M., ucca, S., Gabriella, M., De Angelis, C. & Magni, P. (2019). A synthetic close-loop controller circuit for the regulation of an extracellular molecule by engineered bacteria. IEEE Trans Biomed Circuits Syst. 2019 Feb; 13(1):248-258.
https://doi.org/10.1109/TBCAS.2018.2883350
pH optimization for the aerobical production of itaconic acid catalyzed by Aspergillus terreus in a LAMBDA MINIFOR bioreactor: Batch, working volume 1.8 L, medium with 120 g/L glucose substrate, 37 °C, pH 3 - pH 2.5 - pH 4 - pH 3 - pH 2.5, stirrer 2 Hz, aeration 1.5 L (STP)/min.
Komáromy, K., Bakonyi, P., Kucska, A., Tóth, G., Gubicza, L., Bélafi-Bakó, K. & Nemestóthy, N. (2019). Optimized pH and Its Control Strategy Lead to Enhanced Itaconic Acid Fermentation by Aspergillus terreus on Glucose Substrate. Fermentation 2019, 5(2), 31
https://doi.org/10.3390/fermentation5020031
Optimización del pH para la producción aeróbica de ácido itacónico catalizada por Aspergillus terreus en un biorreactor LAMBDA MINIFOR: Lote, volumen de medio 1.8 L, medio que contiene 120 g/L de glucosa, 37 °C, pH 3 - pH 2.5 - pH 4 - pH 3 - pH 2.5, agitación 2 Hz, aireación 1.5 L(TPS)/min.
Faqri, A. F., Hayder, N.H. & Hashim, A.J. (2019). Lab-scale production of Rhamnolipid by Pseudomonas Aeruginosa A3 and study its synergistic effect with certain antibiotics against some pathogenic bacteria. Iraqi Journal of Agricultural Sciences –2019:50(5):1290-1301.
https://doi.org/10.13140/RG.2.2.10802.35520
2018:
Se desarrolló un método de producción de pro-siRNA a gran escala en un biorreactor LAMBDA MINIFOR para la producción de alto rendimiento de pro-siRNA
Kaur, G., Cheung, H. C., Xu, W., Wong, J. V., Chan, F. F., Li, Y., McReynolds, L. & Huang, L. (2018). Milligram scale production of potent recombinant small interfering RNAs in Escherichia coli. Biotechnology and Bioengineering, 115(9), 2280-2291.
https://doi.org/10.1002/bit.26740
El fermentador de laboratorio LAMBDA MINIFOR se utilizó para la producción de bioetanol en condiciones optimizadas: 3.5 litros de medio con S. cerevisiae activado (inoculación 2 % (v/v), DO= 0.5, 1.5×10E8 UFC/mL) en fermentación aerobia durante 24 h a 30 °C, luego en condiciones anaerobias durante 70 h a 30 °C.
Hayder, N. H., Flayeh, H. M., & Ahmed, A. W. (2018). Optimization of bioethanol production from biodegradable municipal solid waste using response surface methodology (RSM). Journal of Engineering and Sustainable Development, 22(1), 47-64.
https://www.iasj.net/iasj/download/28dcbea4ab5f5ba8 (2024 Feb. 05)
2017:
Comparación de la producción experimental y teórica de biogás. 2 litros de inóculo se incubaron anaeróbicamente a 35 °C durante 1 mes en el biorreactor LAMBDA MINIFOR
El Asri, O., & Afilal, M. E. (2018). Comparison of the experimental and theoretical production of biogas by monosaccharides, disaccharides, and amino acids. International Journal of Environmental Science and Technology, 15(9), 1957-1966.
https://doi.org/10.1007/s13762-017-1570-1
Evaluación del efecto de la carencia de azúcares bajo varias condiciones de oxigenación O2 en células aisladas de vegetales y cultivadas en el LAMBDA MINIFOR
Victor, B. M. M., Ampofo-Asiama, J., Hertog, M., Geeraerd, A. H., & Nicolai, B. M. (2017). Metabolic profiling reveals a coordinated response of isolated lamb's (Valerianella locusta, L.) lettuce cells to sugar starvation and low oxygen stress. Postharvest Biology and Technology, 126, 23-33.
https://doi.org/10.1016/j.postharvbio.2016.12.004
Fermentadores LAMBDA MINIFOR utilizados como digestores anaerobios agitados de flujo continuo (CSTR) para la digestión anaerobia de residuos sólidos orgánicos.
Nakasima-López, M., Taboada-González, P., Aguilar-Virgen, Q., & Velázquez-Limón, N. (2017). Adaptación de inóculos durante el arranque de la digestión anaerobia con residuos sólidos orgánicos. Información tecnológica, 28(1), 199-208.
https://dx.doi.org/10.4067/S0718-07642017000100020
Se estudió el efecto de diferentes temperaturas sobre la inanición de azúcar en células aisladas de hortalizas de hoja verde frescas en el biorreactor LAMBDA MINIFOR
Mbong, V. B. M., Ampofo-Asiama, J., Hertog, M. L., Geeraerd, A. H., & Nicolai, B. M. (2017). The effect of temperature on the metabolic response of lamb’s lettuce (Valerianella locusta,(L), Laterr.) cells to sugar starvation. Postharvest Biology and Technology, 125, 1-12.
https://doi.org/10.1016/j.postharvbio.2016.10.013
Cultivo de células de hibridoma murino en el bioreactor-fermentador LAMBDA MINIFOR
Valdés, R., Aragón, H., González, M., Hernández, D., Geada, D., Goitizolo, D., Ferro, W., Pérez, A., García, J., Masforrol, Y, Aguilar, P., Márquez, G., LaO, M., González, T., Calvo, Y., Hernández, A., Menéndez, G. & Tamayo, A. (2017). Mouse Hybridoma Cell Culture in a Protein-Free Medium Using a Bio-Mimicking Fish-Tail Disc Stirred Bioreactor. BioProcessing Journal, 16(1).
https://doi.org/10.12665/J161.Valdes
2016:
Robusta producción de etanol celulósico a partir de bagazo de la caña de azúcar con Saccharomyces cerevisiae ATCC 20602 en el bioreactor LAMBDA MINIFOR bajo condiciones aeróbicas y anaeróbicas con medición controlada del potencial Redox
Jabasingh, S. A., Lalith, D., Prabhu, M. A., Yimam, A., & Zewdu, T. (2016). Catalytic conversion of sugarcane bagasse to cellulosic ethanol: TiO2 coupled nanocellulose as an effective hydrolysis enhancer. Carbohydrate polymers, 136, 700-709.
https://doi.org/10.1016/j.carbpol.2015.09.098
2015:
El sistema de biorreactor LAMBDA MINIFOR se utiliza como boca artificial para el cultivo de biopelículas
LA BOCA ARTIFICIAL DE DENTAID UNA REVOLUCIÓN EN INVESTIGACIÓN BUCODENTAL
DENTAID EXPERTISE, PUBLICACIÓN PARA PROFESIONALES DE LA ODONTOLOGÍA, NÚM. 18.
https://aprenderly.com/doc/3463742/la-boca-artificial-de-dentaid-una-revolución-en-investiga…?page=5 (2024 Feb. 05)
Expresión de la proteína S. pyogenes Cas9 usando el bioreactor LAMBDA MINIFOR con vaso de 3L controlado por una computadora en modo de operación batch seguida de una alimentación exponencial
Ménoret, S., De Cian, A., Tesson, L., Remy, S., Usal, C., Boulé, J. B., Boix, C., Fontanière, S., Crénéguy, A., Nguyen, T.H., Brusselle, L., Thinard, R., Gauguier, D., Concordet, J.-P., Cherifi, Y., Fraichard, A., Giovannangeli, C. & Anegon, I. (2015). Homology-directed repair in rodent zygotes using Cas9 and TALEN engineered proteins. Scientific reports, 5(1), 14410.
https://doi.org/10.1038/srep14410
Fermentación de microorganismos diseñados en biorreactor a escala de laboratorio LAMBDA MINIFOR para la conversión eficiente de lactosa en etanol
Pasotti, L., Zucca, S., Casanova, M., Massaiu, I., Mazzini, G., Micoli, G., Calvio, C., Cusella de Angelis, M.G. & Magni, P. (2015, August). Methods for genetic optimization of biocatalysts for biofuel production from dairy waste through synthetic biology. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 953-956). IEEE.
https://doi.org/10.1109/EMBC.2015.7318521
Un modelo de biofilme celular de flujo con 6 especies se desarrolló y se cultivaron las bacterias en el fermentador LAMBDA MINIFOR para evaluar el desarrollo del biofilme bajo condiciones de fuerzas de corte y flujo
Salli, K. M., & Ouwehand, A. C. (2015). The use of in vitro model systems to study dental biofilms associated with caries: a short review. Journal of oral microbiology, 7(1), 26149.
https://doi.org/10.3402/jom.v7.26149
Cuantificación de las proteínas ribosomales (RPs) a partir de células de levaduras cultivadas en el MINIFOR y células madres embrionarias de ratón (ESC) para estudiar la estequeometría del core o núcleo de las RPs
Slavov, N., Semrau, S., Airoldi, E., Budnik, B., & van Oudenaarden, A. (2015). Differential stoichiometry among core ribosomal proteins. Cell reports, 13(5), 865-873.
https://doi.org/10.1016/j.celrep.2015.09.056
Harvard University, USA; Broad Institute of MIT and Harvard, USA and Hubrecht Institute, Netherlands.
2014:
Cultivo de la microalga (Chlorella vulgaris Beyerinck) en el bioreactor de laboratorio LAMBDA MINIFOR
Heitur, H. (2014). Mikrovetika Chlorella vulgaris Beyerincki kasvatamine CO2 sidumise eesmärgil (Master's thesis).
https://hdl.handle.net/10492/1842 (2024 Feb. 05)
Eesti Maaülikool (Estonian University of Life Sciences), Estonia.
Crecimiento de cultivos de levaduras (DBY12007) en el LAMBDA MINIFOR en estado estacionario para estudiar la glicólisis aerobia y el flujo de energía
Slavov, N., Budnik, B. A., Schwab, D., Airoldi, E. M., & van Oudenaarden, A. (2014). Constant growth rate can be supported by decreasing energy flux and increasing aerobic glycolysis. Cell reports, 7(3), 705-714.
https://doi.org/10.1016/j.celrep.2014.03.057
Massachusetts Institute of Technology, USA; Harvard University, USA; Hubrecht Institute, Royal Netherlands Academy of Arts and Sciences and University Medical Center Utrecht, Netherlands and Princeton University, USA.
Fermentación en lote, selectiva y no-selectiva de extractos de dátiles usando Saccharomyces cerevisiae (línea comercial usada en reposterías (línea silvestre), líneas glucosa selectivas ATCC 36858 y ATCC 36859) en el fermentador LAMBDA MINIFOR
Putra, M. D., Abasaeed, A. E., Zeinelabdeen, M. A., Gaily, M. H., & Sulieman, A. K. (2014, April). Selective fermentation of pitted dates by S. cerevisiae for the production of concentrated fructose syrups and ethanol. In Journal of Physics: Conference Series (Vol. 495, No. 1, p. 012034). IOP Publishing.
https://doi.org/10.1088/1742-6596/495/1/012034
King Saud University, Chemical Engineering Department, Saudi Arabia
La respuesta de estrés metabólico en cultivo de células de tomate (Lycopersicum esculentum) a baja concentración de oxígeno usando el bioreactor LAMBDA MINIFOR.
Ampofo‐Asiama, J., Baiye, V. M. M., Hertog, M. L. A. T. M., Waelkens, E., Geeraerd, A. H., & Nicolai, B. M. (2014). The metabolic response of cultured tomato cells to low oxygen stress. Plant Biology, 16(3), 594-606.
https://doi.org/10.1111/plb.12094
KU Leuven, Belgium; Flanders Centre of Postharvest Technology (VCBT), Leuven, Belgium.
El bioreactor LAMBDA MINIFOR es usado para crecer bacterias orales (Streptococcus oralis, Actinomyces naeslundii, Veillonella parvula, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans y Porphyromonas gingivalis) bajo condiciones de plakton
Blanc, V., Isabal, S., Sanchez, M. C., Llama‐Palacios, A., Herrera, D., Sanz, M., & León, R. (2014). Characterization and application of a flow system for in vitro multispecies oral biofilm formation. Journal of Periodontal Research, 49(3), 323-332.
https://doi.org/10.1111/jre.12110
DENTAID S. L., Cerdanyola del Vallès, Spain; ETEP Research Group, University Complutense of Madrid, Spain.
LAMBDA MINIFOR Bioreactor utilizado para la expresión de proteína recombinante (Chemokines) en E. coli.
Kramp, B. (2014). Establishing the interaction between the CC chemokine ligand 5 and the receptors CCR1 and CCR (Doctoral dissertation, Aachen, Techn. Hochsch., Diss., 2013).
https://core.ac.uk/download/pdf/36589112.pdf (2024 Feb. 05)
RWTH Aachen, Germany.
2013:
Desarrollo de modelo de biofilm (S. oralis, A. naeslundii, V. parvula, F. nucleatum, A. actinomycetemcomitans y P. gingivalis) en flujo continuo con un biorreactor LAMBDA MINIFOR 0.4L.
Suárez Soto, I. (2013). Desarrollo del modelo de boca artificial en flujo continuo en el biorreactor Lambda Minifor. Ene, 10, 41.
https://docta.ucm.es/rest/api/core/bitstreams/39d8318b-a49b-4cb3-a164-e4faab18f52b/content (2024 Feb. 05)
Recombinant expression of the Met-CCL5, protease resistant CXCL12 (S4V) and F1-CX3CL1 in E. coli using LAMBDA MINIFOR fermenter/bioreactor to study their role in Cardiovascular disease (CVD)
Projahn, D. (2013). Generation, function and therapeutic application of chemotactic cytokines in cardiovascular diseases (Doctoral dissertation, Aachen, Techn. Hochsch., Diss., 2013).
https://publications.rwth-aachen.de/record/229207/files/4840.pdf (2024 Feb. 05)
RWTH Aachen, Germany.
Expresión recombinante de Met-CCL5, proteasa resistente CXCL12 (S4V) y F1-CX3CL1 en E. coli para estudiar su rol en la enfermedad cardiovascular (CVD).
Machado Roque, A. I. (2013). Protein scaffolds for cell culture (Doctoral dissertation, Newcastle University).
URI: https://hdl.handle.net/10443/1843 (2024 Feb. 05)
Newcastle University, UK.
Crecimiento controlado de Staphylococcus aureus bajo diversas concentraciones de BAC (cloruro de benzalconio) en el fermentador LAMBDA MINIFOR
Cervinkova, D., Babak, V., Marosevic, D., Kubikova, I., & Jaglic, Z. (2013). The role of the qacA gene in mediating resistance to quaternary ammonium compounds. Microbial Drug Resistance, 19(3), 160-167.
https://doi.org/10.1089/mdr.2012.0154
Veterinary Research Institute, Brno, Czech Republic.
2012:
Producción efectiva de biobutanol a partir de desechos agrícolas
Mezule, L., Tihomirova, K., Nescerecka, A., & Juhna, T. (2012). Biobutanol production from agricultural waste: A simple approach for pre-treatment and hydrolysis. Latvian Journal of Chemistry, 51(4), 407.
https://doi.org/10.2478/v10161-012-0028-5
2011:
Producción de bioetanol usando levaduras (S. cerevisiae) en el fermentador LAMBDA MINIFOR
Burešová, I., & Hřivna, L. (2011). Effect of wheat gluten proteins on bioethanol yield from grain. Applied Energy, 88(4), 1205-1210.
https://doi.org/10.1016/j.apenergy.2010.10.036
2010:
Fermentación anaeróbica del componente de glucosa en los extractos de dátiles por la levadura Saccharomyces cerevisiae
Gaily, M. H., Elhassan, B. M., Abasaeed, A. E., & Al-Zahrani, S. M. (2010). A direct process for the production of high fructose syrups from dates extracts. International Journal of Food Engineering, 6(3).
https://doi.org/10.2202/1556-3758.1879
King Saud University, Saudi Arabia; University of Khartoum, Sudan
Estudio del potencial de los tallos de tabaco arbóreo (Nicotiana glauca r. Grah.) como materia prima de bioetanol en el fermentador LAMBDA MINIFOR
Sánchez, F., Curt, M. D., Barreiro, M., Fernández, J., Agüera, J. M., Uceda, M., & Zaragoza, G. (2010). TREE TOBACCO (NICOTIANA GLAUCA R. GRAH.) STEMS AS A BIOETHANOL FEEDSTOCK. 18th European Biomass Conference and Exhibition, 3-7 May 2010, Lyon, France.
Dpt. Producción Vegetal: Botánica y Protección Vegetal. Universidad Politécnica de Madrid (UPM), Madrid, Spain
2009:
Determinación del potencial alcoholígeno de carbohidratos no celulósicos procedentes de cladodios de higo chumbo mediante fermentación con la levadura Saccharomyces cerevisiae (cepas comerciales).
Sánchez, F., Curt, M. D., Fernández, J., Agüera, J. M., Uceda, M., & Zaragoza, G. (2009). Bioethanol production from prickly pear (Opuntia ficus-indica (L.) Mill.) cladodes. In Proc. 17th European Biomass Conference. Pub. ETA-Florence Renewable Energies & WIP-Renewable Energies. ISBN (pp. 978-88).
Dpt. Producción Vegetal: Botánica y Protección Vegetal. Universidad Politécnica de Madrid (UPM), Madrid, Spain
<b< p="">
Do you sell/ship to the USA?
Yes, we do supply our instruments directly with door-to-door delivery option by the parcel services to the USA.
What is the availability of the product?
We have the instruments in stock. We would just have to configure the instruments according to your requirements and perform quality control before shipping.
Is there a warranty?
We offer a 2 year warranty for MINIFOR fermentor / bioreactor and 5 year warranty for the PRECIFLOW & MULTIFLOW peristaltic pumps.
Does this fermentor work on both mammalian cells and yeast cells?
Yes, MINIFOR fermentor and bioreactor can be used for mammalian and yeast cell cultures (More information at www.fermentor.net/applications).
Is there flexibility in the top plate to add or remove probes?
Yes, MINIFOR has free ports in the headspace for the additional probes (sensors). Multiple ports and other effective solutions in the fermentation glass vess make the MINIFOR configuration equivalent to 16 to 22 classical ports (it is possible to increase the number of ports – custom made solution)
Is the equipment suitable for use in pure / mixed culture?
Yes, MINIFOR is suitable for pure as well as mixed culture. The stirrer is strong and can easily be adapted according to the types of cultures and working volumes.
Why is MINIFOR perfectly suitable for parallel processes?
Each unit stays independent as it is equipped with a control panel and display and at a single glance shows the parameter values. All parameters are regulated locally inside each fermenter-bioreactor unit.
This allows fast and precise parameter regulation and never having to worry about leaving a vessel unattended. Further advantage is that in case there are problems with one unit, the other units will still keep running.
How important is the slowdown in parameter regulation while running 12 bioreactors in parallel?
An important aspect to consider – which, however, does not play a role in the LAMBDA MINIFOR parallel system because each MINIFOR fermenter comes with its proper regulation unit that measures and controls all parameters locally. As a consequence the quality of the measurement and regulation is not affected by long transmission times and dead times in regulation.
How much space is required for the MINIFOR unit?
Footprint: approximately a sheet of paper
Dimensions: 22 cm x 38 cm x 40 cm (W x H x D)
 Módulo de pesada para cultivo continuo
Mostrar detalles
Módulo de pesada para cultivo continuo
Mostrar detalles
 Unidad de medición de potencial REDOX
Mostrar detalles
Unidad de medición de potencial REDOX
Mostrar detalles
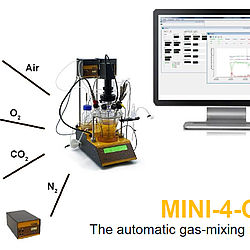 MINI-4-GAS Módulo automático de mezcla de gases
Mostrar detalles
MINI-4-GAS Módulo automático de mezcla de gases
Mostrar detalles
 El sistema más pequeño de control anti-espuma
Mostrar detalles
El sistema más pequeño de control anti-espuma
Mostrar detalles
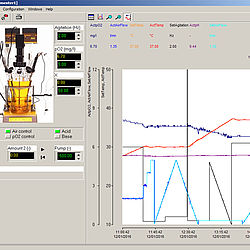 FNet - Programa de fermentación
Mostrar detalles
FNet - Programa de fermentación
Mostrar detalles
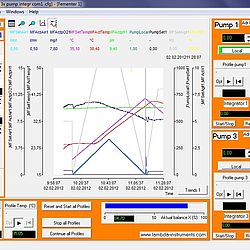 Programa de fermentación Industrial SIAM
Mostrar detalles
Programa de fermentación Industrial SIAM
Mostrar detalles
 Módulo del programa del controlador de gases
Mostrar detalles
Módulo del programa del controlador de gases
Mostrar detalles
 LAMBDA OXÍMETRO
Medición de la concentración de O2 en el gas de salida
Mostrar detalles
LAMBDA OXÍMETRO
Medición de la concentración de O2 en el gas de salida
Mostrar detalles
 LAMBDA CARBOXÍMETRO
Medición de la concentración de CO2 en el gas de salida
Mostrar detalles
LAMBDA CARBOXÍMETRO
Medición de la concentración de CO2 en el gas de salida
Mostrar detalles
 LAMBDA METÁMETRO
Medición de la concentración de CH4 en el gas de salida
Mostrar detalles
LAMBDA METÁMETRO
Medición de la concentración de CH4 en el gas de salida
Mostrar detalles
 Additional PRECIFLOW pump line
PRECIFLOW pump 0-600 ml/h, reagent bottle with pipes, fittings, filter, tubing
Mostrar detalles
Additional PRECIFLOW pump line
PRECIFLOW pump 0-600 ml/h, reagent bottle with pipes, fittings, filter, tubing
Mostrar detalles
 Additional MULTIFLOW pump line
MULTIFLOW pump, reagent bottle with pipes, fittings, filter and tubing
Mostrar detalles
Additional MULTIFLOW pump line
MULTIFLOW pump, reagent bottle with pipes, fittings, filter and tubing
Mostrar detalles